Nutrient concentrations in coarse and fine woody debris of Populus tremuloides Michx.-dominated forests, northern Minnesota, USA
Klockow P. A., D'Amato A. W., Bradford J. B., Fraver S. (2014). Nutrient concentrations in coarse and fine woody debris of Populus tremuloides Michx.-dominated forests, northern Minnesota, USA. Silva Fennica vol. 48 no. 1 article id 962. https://doi.org/10.14214/sf.962
Highlights
- We examine effects of size, species, and decay on woody debris nutrient concentrations
- Results indicate wide variation in nutrient concentrations across the factors examined
- Fine woody debris nutrient concentrations were greater than in coarse woody debris
- Coarse woody debris nutrient concentrations generally increased as decay progressed
- Results suggest high fine woody debris stocks can represent an important nutrient source.
Abstract
Contemporary forest harvesting practices, specifically harvesting woody biomass as a source of bioenergy feedstock, may remove more woody debris from a site than conventional harvesting. Woody debris, particularly smaller diameter woody debris, plays a key role in maintaining ecosystem nutrient stores following disturbance. Understanding nutrient concentrations within woody debris is necessary for assessing the long-term nutrient balance consequences of altered woody debris retention, particularly in forests slated for use as bioenergy feedstocks. Nutrient concentrations in downed woody debris of various sizes, decay classes, and species were characterized within one such forest type, Populus tremuloides Michx.-dominated forests of northern Minnesota, USA. Nutrient concentrations differed significantly between size and decay classes and generally increased as decay progressed. Fine woody debris (≤ 7.5 cm diameter) had higher nutrient concentrations than coarse woody debris (> 7.5 cm diameter) for all nutrients examined except Na and Mn, and nutrient concentrations varied among species. Concentrations of N, Mn, Al, Fe, and Zn in coarse woody debris increased between one and three orders of magnitude, while K decreased by an order of magnitude with progressing decay. The variations in nutrient concentrations observed here underscore the complexity of woody debris nutrient stores in forested ecosystems and suggest that retaining fine woody debris at harvest may provide a potentially important source of nutrients following intensive removals of bioenergy feedstocks.
Keywords
coarse woody debris;
fine woody debris;
Populus tremuloides;
nutrient concentrations;
bioenergy feedstock harvesting;
Great Lakes
-
Klockow,
Department of Forest Resources, University of Minnesota, St. Paul, MN 55108, USA
E-mail
klock039@umn.edu

- D'Amato, Department of Forest Resources, University of Minnesota, St. Paul, MN 55108, USA E-mail damato@umn.edu
- Bradford, US Geological Survey, Southwest Biological Science Center, Flagstaff, AZ 86001, USA E-mail jbradford@usgs.gov
- Fraver, School of Forest Resources, University of Maine, Orono, ME 04469, USA E-mail shawn.fraver@maine.edu
Received 8 July 2013 Accepted 15 January 2014 Published 6 February 2014
Views 168807
Available at https://doi.org/10.14214/sf.962 | Download PDF

1 Introduction
Downed woody debris is a critical component of forested ecosystems worldwide (Harmon et al. 1986; Laiho and Prescott 2004). Large woody debris, typically termed ‘coarse’ woody debris (CWD), provides habitat and protection for a wide array of organisms (Bunnell and Houde 2010), notably deadwood-dependent insects and fungi (Siitonen 2001). CWD provides suitable substrates, or ‘nurse logs,’ for regenerating vascular plants (Bolton and D’Amato 2011), microclimates that protect organisms from extreme environmental conditions (Åström et al. 2005), inputs of organic matter into the forest floor and soil (Krzyszowska-Waitkus et al. 2006), and is important for controlling soil erosion and influencing hydrologic and geomorphologic processes (Harmon et al. 1986). Although less studied, smaller woody debris, termed ‘fine’ woody debris (FWD) has recently received more attention, and its function in forested ecosystems is becoming better recognized (Juutilainen et al. 2011). For example, FWD provides important microclimates for bryophytes (Dynesius et al. 2008), substrate for fungi (Brazee et al. 2012), and habitat for deadwood-dependent insects (Jonsell et al. 2007).
The role of woody debris in forest nutrient cycling has been widely studied (Pastor and Bockheim 1984; Laiho and Prescott 2004; Saunders et al. 2011). Nutrient concentrations in living bole wood depend largely on species and site factors (Pallardy 2008). Initially, CWD contains low nutrient concentrations, similar to those of live wood, and concentrations typically increase with time (Alban and Pastor 1993; Herrmann and Prescott 2008; Saunders et al. 2011). However, this trend varies considerably depending on woody debris species, particular nutrients under study, and initial nutrient concentrations, as well as climate, soil type, and the decomposer community (Maser and Trappe 1984; Brais et al. 2006; Kuehne et al. 2008; Herrmann and Bauhus 2012; Gonzalez-Polo et al. 2013; Lombardi et al. 2013). As CWD decays it becomes fragmented, nutrients are leached, organic matter is broken down by various decomposers, and the original material eventually becomes incorporated into the forest floor and soil (Harmon et al. 1986).
In contrast to CWD, FWD contains relatively high nutrient concentrations, because live twigs and branches are more nutrient-rich than living bole wood (Whittaker et al. 1979; Lang et al. 1982; Miller 1983). FWD is found in large quantities after forest disturbances, particularly following harvest, and tends to decay more rapidly than larger CWD (Moroni and Ryan 2010). Given its high nutrient concentrations, ubiquitous presence following disturbance, and relatively high decomposition rates, FWD can quickly return high amounts of nutrients to the forest floor and mineral soil following disturbance (Miller 1983).
Because of its prevalence in the upper Great Lakes region of North America and the ease with which it regenerates, P. tremuloides forests are under strong consideration for sustained use as bioenergy feedstocks (Rittenhouse et al. 2012; Klockow et al. 2013). Harvesting woody debris as bioenergy feedstocks has been shown to reduce tree growth in Scandinavian forests for several decades post-harvest (Helmisaari et al. 2011). Quantifying nutrient concentrations in CWD and FWD on similarly harvested sites will provide crucial information for predicting future nutrient stocks and assessing possible reductions in site productivity. However, few studies have focused on nutrient concentrations of both CWD and FWD in these forests (but see Alban and Pastor 1993; Miller 1983).
Our objective was to characterize nutrient concentrations within both CWD and FWD across various species and decay stages in the prevalent P. tremuloides forests of northern Minnesota using sites established as part of a long-term study of the impacts of bioenergy feedstock harvesting. We focus on the predominant species in these forests, specifically, four hardwood species, P. tremuloides, Acer rubrum L., Betula papyrifera Marshall, and Fraxinus nigra Marshall and one softwood species, Abies balsamea (L.) Mill. Results from this study will provide comprehensive baseline knowledge important for informing future research as well as management decisions regarding the role of CWD and FWD in forest nutrient cycling following repeated bioenergy feedstock harvests, which are expected to increase dramatically in this and other portions of north temperate regions.
2 Materials and methods
2.1 Study sites
Three sites located in St. Louis County, Minnesota, USA, near the towns of Independence, Minnesota (47°00´N, 92°24´W); Melrude, Minnesota (47°15´N, 92°19´W); and Orr, Minnesota (48°09´N, 92°59´W) were selected for sampling. Soils generally consisted of loams derived from glacial till. The site near Independence contained stony to very stony loams and sandy loams, while soils near Melrude and Orr were silt loams and loams. More specifically, the Independence site contained coarse-loamy, isotic, frigid Aquic Dystric Eutrudepts; the Melrude site contained fine-loamy, mixed, superactive, frigid Aquic Glossudalfs and fine-loamy, mixed, superactive, frigid Typic Glossaqualfs; and the Orr site contained fine, smectitic, frigid Aquic Glossudalfs and fine, smectitic, frigid Oxyaquic Glossudalfs. Climate in these areas is continental with a mean temperature of –16 °C in January and 26 °C in July. Mean annual precipitation ranges between 660–710 mm, about 75% of which occurs between the months of May and October. Stands were mesic, dominated by P. tremuloides, originated from clearcutting, and ranged in age from 56 to 68 years. Over the winter of 2009–10, the study sites were subjected to an array of bioenergy feedstock harvests consisting of tree removal with varying levels of slash and live-tree retention. For a more detailed description of these harvests see Klockow et al. (2013).
2.2 Study design and sample collection
Samples, collected summer 2010, consisted of FWD (≤ 7.5 cm diameter) and CWD (> 7.5 cm diameter) obtained by cutting downed wood with a handsaw. These size classes were based on Brown (1974). Samples were selected from open areas of recently harvested sites as well as the unharvested forest matrix adjacent to the harvested areas. Unharvested areas were control plots for a larger study examining the impacts of bioenergy harvests and were identical in composition, structure, and history to harvested areas. Since harvest and site-preparation operations can disturb the detrital layer of a forest and particularly any highly-decayed woody debris present (Hautala et al. 2004), our well-decayed samples (decay class 3–5) tended to be more prevalent in and were typically selected from the unharvested forest matrix surrounding recent harvests while less-decayed woody debris samples (decay classes 1 and 2) tended to be selected from retained slash in recently harvested areas. Additionally, species were targeted based on documented pre-harvest prevalence in the harvested stands and adjacent unharvested forest matrix. Depending on diameter, either a disk (CWD), small segment of branch (FWD), or small group of twigs (FWD) was removed. We selected samples both directly in contact with the ground (30% of samples) and elevated above the ground (70% of samples) in order to better capture the potential range of nutrient concentrations of woody debris in various positions. Samples in contact with the ground tended to be highly decayed material (decay classes 4 and 5) or smaller sections of less-decayed logs (decay classes 1 and 2) in clearcut areas. Measurements for both FWD and CWD included diameter, species or wood type (hardwood [HW] and softwood [SW]), and decay class.
Sampled species were chosen based on calculated importance values in the overstory of the pre-harvest stand and are common overstory species throughout the upper Great Lakes region. Specifically, five species were sampled for both FWD and CWD including P. tremuloides, A. balsamea, A. rubrum, B. papyrifera, and F. nigra. Decay was assigned based on two systems, one for CWD and another for FWD. For CWD, decay state was assigned based on a five-class system derived from Sollins (1982) with emphasis placed on status of bark, structural integrity, and branches. For FWD, decay state was assigned based on a two-class system defined as follows: Class 1 – bark intact, twigs and smaller branches pliable or easily bent without breaking, and foliage may still be present; Class 2 – bark sloughing but generally still present, twigs and smaller branches easily snapped when bent, and foliage absent.
In all cases, an attempt to collect bark was made. Decay class 4 CWD samples were identified to HW or SW only, due to difficulty in species identification. For decay class 5 CWD, species and wood type were often indistinguishable and were therefore not recorded. Samples in advanced stages of decay were wrapped in plastic and duct tape to stabilize loose pieces. After removal, each sample was placed inside a labeled, re-sealable plastic storage bag, immediately returned to the laboratory, and frozen until processing.
2.3 Sample processing and nutrient analyses
A total of 180 CWD samples were collected across all three sites. Sample totals included 10 samples of each of the five sampled species in decay classes 1–3 (150 total samples), 10 samples each of HW or SW in decay class 4 (20 total samples), and 10 samples in decay class 5. A total of 100 FWD samples were collected across all three sites. Sample totals for FWD included 10 samples of each of the five sampled species in each of the two decay classes (50 total samples in decay class 1and 50 total samples in decay class 2). FWD samples were selected such that at least three samples of each species in each decay class were within each of the following three size classes: ≤1 cm (at the largest end), > 1 to ≤ 2.5 cm, and > 2.5 to ≤ 7.5 cm. Preliminary analyses indicated that site did not significantly affect nutrient concentrations and was excluded from further analyses.
Samples were dried at 70 °C until a constant mass was reached. After drying, pie-shaped wedges were cut from CWD and larger FWD samples in an attempt to obtain representative proportions of bark-to-pith for analyses. Cut samples and smaller FWD were then ground and homogenized twice in Wiley mills.
Percent total carbon (C) and nitrogen (N) concentration were determined for each sample using a LECO Truspec CHN Macro analyzer (LECO Corporation, St. Joseph, Michigan). Concentrations of phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sodium (Na), manganese (Mn), aluminum (Al), boron (B), copper (Cu), iron (Fe), and zinc (Zn) were determined by Inductively-Coupled Plasma Atomic Emission Spectrometry (ICP-AES) analysis with a Perkin Elmer Optima 3000 ICP Spectrometer (Perkin Elmer, Inc., Waltham, Massachusetts). Within this study, we define macronutrients as C, N, P, K, Ca, and Mg and micronutrients as Na, Mn, Al, B, Cu, Fe, and Zn.
2.4 Statistical analyses
Analysis of variance (ANOVA) was used to determine the effects of size, species, and decay class on nutrient concentrations using PROC GLM in SAS (SAS Institute, Inc). Because most nutrient data were highly skewed with non-normal residuals and heterogeneous variances and therefore failed to meet ANOVA assumptions, dependent variables were transformed using natural logarithm, square root, or inverse functions. When these transformed data still failed to meet the necessary assumptions, rank transformations were applied (Conover and Iman 1981), which permit the testing of interactions and post-hoc pairwise comparisons. The Tukey-Kramer method was employed to test for differences between species-decay class combinations and between sizes of woody debris (CWD vs. FWD).
Ideal characterization of CWD nutrient concentrations would include analyses of each species across a complete range of decay, in our case, five stages of decay. However, limitations with identification of species and wood type in advanced decay stages made this unfeasible. Therefore, we present nutrient concentrations of CWD analyzed in two separate scenarios such that species are accounted for whenever possible. Analyses conducted for CWD included the effects of each species in decay classes 1 through 3 and the effects of decay classes 1 through 5 alone on nutrient concentrations. Analyses for FWD included the effects of each species in decay classes 1 and 2 on nutrient concentrations. In addition, we compared nutrient concentrations of decay class 1 CWD with decay class 1 FWD to determine if nutrient concentrations differed based on size of woody debris material. All significance testing was at α = 0.05 level and results are presented in non-transformed format.
3 Results
3.1 Fine woody debris vs. coarse woody debris
Diameters of FWD ranged from 0.5 cm (at the large end) to 7.0 cm with a mean diameter of 2.6 ± 0.2 cm. CWD diameters ranged from 7.6 cm to 26.0 cm with a mean diameter of 12.9 ± 0.3 cm. The effect of size (CWD vs. FWD) was highly significant for nearly all nutrient concentrations in decay class 1 material. In particular, nutrient concentrations in FWD were significantly greater than those found in CWD for all nutrients in decay class 1 with the exception of Na and Mn, which showed no significant difference (Table 1).
3.2 Fine woody debris nutrient concentrations by species and decay class
Nutrient concentrations in FWD varied significantly by species and decay class (Table 1, 2). Species alone was a significant factor for some nutrient concentrations including C, N, Ca, Na, Al, B, Cu, Fe, and Zn (Table 2). Of these nutrients, Zn and C had the most differences among species while the fewest differences occurred in Fe (Table A1). Among all nutrients in FWD, B. papyrifera tended to have the highest nutrient concentrations and A. rubrum tended to have the lowest (Table 1). Decay class alone was a significant factor for a few nutrients, particularly, C, Al, and Fe which all increased significantly (Table 1, 2). Generally, nutrient concentrations tended to increase from decay class 1 to 2 with a few nutrients decreasing (P, K, and B; Table 1).
| Table 1. Mean decay class 1 fine woody debris (FWD) and coarse woody debris (CWD) nutrient concentrations pooled across species and FWD nutrient concentrations for each species by decay class. Standard errors are in parentheses. Carbon is presented in percent total (%), while all other nutrients are in mg kg–1. Sample sizes are n = 50 for decay class 1 FWD and CWD nutrient concentrations within each group and n = 10 for FWD nutrient concentrations within each species/decay class combination. Within each row, values with similar letters are not significantly different (p > 0.05) for decay class 1 FWD and CWD nutrient concentrations pooled across species and separately for FWD nutrient concentrations by species and decay class. View in new window/tab. |
| Table 2. Analysis of variance (ANOVA) table for all nutrients analyzed in fine woody debris (FWD) including the main factors and interaction, F-values with degrees of freedom, and probabilities for each nutrient analyzed. Comparisons among species, decay class, and the interaction are presented with significant differences (p ≤ 0.05) highlighted in bold. | ||||||
| Nutrient | Species | Decay class | Species X decay class | |||
| F(4, 90) | p | F(1, 90) | p | F(4, 90) | p | |
| C | 38.11 | <.0001 | 6.28 | 0.0140 | 0.99 | 0.4175 |
| N | 5.26 | 0.0007 | 1.03 | 0.3124 | 1.28 | 0.2840 |
| P | 2.57 | 0.0431 | 27.09 | <.0001 | 3.03 | 0.0215 |
| K | 7.70 | <.0001 | 61.64 | <.0001 | 2.66 | 0.0377 |
| Ca | 6.37 | 0.0001 | 2.52 | 0.1160 | 1.59 | 0.1829 |
| Mg | 5.18 | 0.0008 | 0.32 | 0.5756 | 4.09 | 0.0043 |
| Na | 4.03 | 0.0048 | 1.90 | 0.1718 | 0.95 | 0.4385 |
| Mn | 80.90 | <.0001 | 11.14 | 0.0012 | 4.44 | 0.0026 |
| Al | 8.11 | <.0001 | 23.40 | <.0001 | 1.29 | 0.2804 |
| B | 11.40 | <.0001 | 2.01 | 0.1596 | 1.41 | 0.2361 |
| Cu | 6.52 | 0.0001 | 1.54 | 0.2185 | 1.14 | 0.3431 |
| Fe | 3.20 | 0.0167 | 20.86 | <.0001 | 1.69 | 0.1598 |
| Zn | 59.31 | <.0001 | 2.36 | 0.1279 | 0.20 | 0.9357 |
Significant species and decay-class interactions were detected in FWD for P, K, Mg, and Mn (Table 2). Specifically, within P. tremuloides, P, K, and Mg decreased from decay class 1 to 2 by 73%, 72%, and 45%, respectively; within F. nigra, K decreased by 63% from decay class 1 to 2; within A. balsamea, Mn increased by 158% from decay class 1 to 2; and within B. papyrifera, Al increased by 443% from decay class 1 to 2 (Table 1). The highest nutrient concentrations among all species and decay classes in FWD occurred within decay class 1 P. tremuloides and decay class 2 B. papyrifera, and the lowest concentrations were found within decay class 1 A. rubrum followed by decay class 1 F. nigra (Table 1).
3.3 Coarse woody debris nutrient concentrations by species and decay class
As with FWD, variation in CWD nutrient concentrations was related to species and decay class, specifically decay class 1 through decay class 3 (Fig. 1, Table A2). The effects of species alone were significant for some CWD nutrient concentrations including N, K, Na, Mn, and Cu (Fig. 1, Table A3, A4). Of these nutrients, K had the most differences among species while Cu had the fewest differences among species (Fig. 1, Table A3, A4). Among all nutrients in CWD, F. nigra tended to have the highest concentration of each nutrient, whereas no particular species consistently had the lowest concentration of each nutrient (Fig. 1, Table A2). Decay class alone had a significant effect on some CWD nutrient concentrations including N, Na, Mn, and Cu (Fig. 1, Table A3, A4). Most nutrients tended to increase from decay class 1 to 3 with a few exceptions (P and K; Fig. 1, Table A2). No significant differences existed between decay class 1 and 2 nutrient concentrations, yet most were significantly different between decay class 1 and 3 and/or decay class 2 and 3 (Fig. 1, Table A3, A4).
The interaction between species and decay class was significant for the majority of CWD nutrients (C, P, Ca, Mg, Al, B, Fe, and Zn; Table A3, A4). Of all nutrients other than C, Ca had the highest concentrations followed by K, while the lowest nutrient concentrations were typically Cu followed by B and Al (Fig. 1, Table A2). More specifically, for each nutrient, decay class 3 F. nigra tended to have the highest nutrient concentrations while decay class 1 A. rubrum and decay class 1 B. papyrifera tended to have the lowest concentration of each nutrient (Fig. 1, Table A2). No N was detected above 24 mg kg–1 in decay class 1 and 2 P. tremuloides as well as decay class 1 A. rubrum (Fig. 1, Table A2), as this was the lowest detectable limit for N.
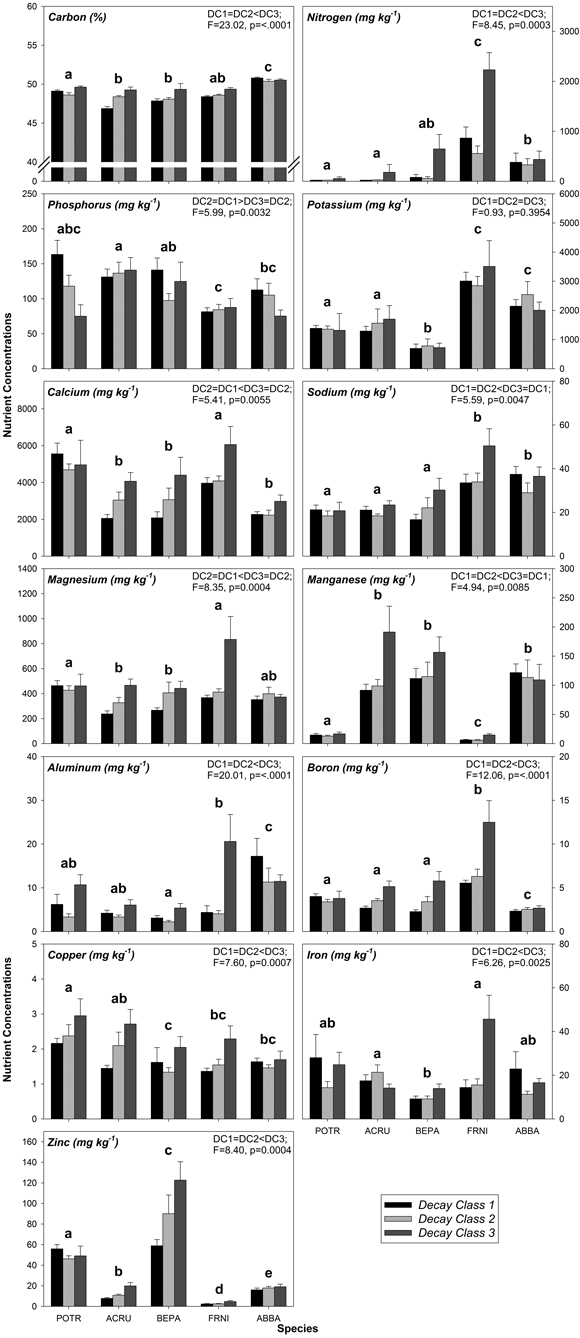
Fig. 1. Mean coarse woody debris (CWD) nutrient concentrations by decay class grouped by species. Similar letters above species groups within a graph indicate no significant differences (p > 0.05) between species, where POTR = Populus tremuloides, ACRU = Acer rubrum, BEPA = Betula papyrifera, FRNI = Fraxinus nigra, and ABBA = Abies balsamea. Data in upper right corner of each graph indicate significant differences (p ≤ 0.05) between decay classes alone, where DC = decay class. Sample sizes within each species/decay class are n = 10.
3.4 Coarse woody debris nutrient concentrations by decay class only
Decay class was highly significant for each nutrient analyzed from decay class 1 to 5 (Fig. 2, Table A5). Generally, nutrient concentrations increased from decay class 1 to 5 with the exception of K, which decreased (Fig. 2, Table A5). A few nutrients worth noting included C which increased before decreasing by 23% from decay class 4 to 5 and P which decreased in concentration before increasing by 409% from decay class 3 to 5 (Fig. 2, Table A5). For N, Mn, Al, Fe, and Zn, nutrient concentrations increased and, for K, decreased by an order of magnitude or more from decay class 3 to 4 and/or decay class 4 to 5 (Fig. 2, Table A5).
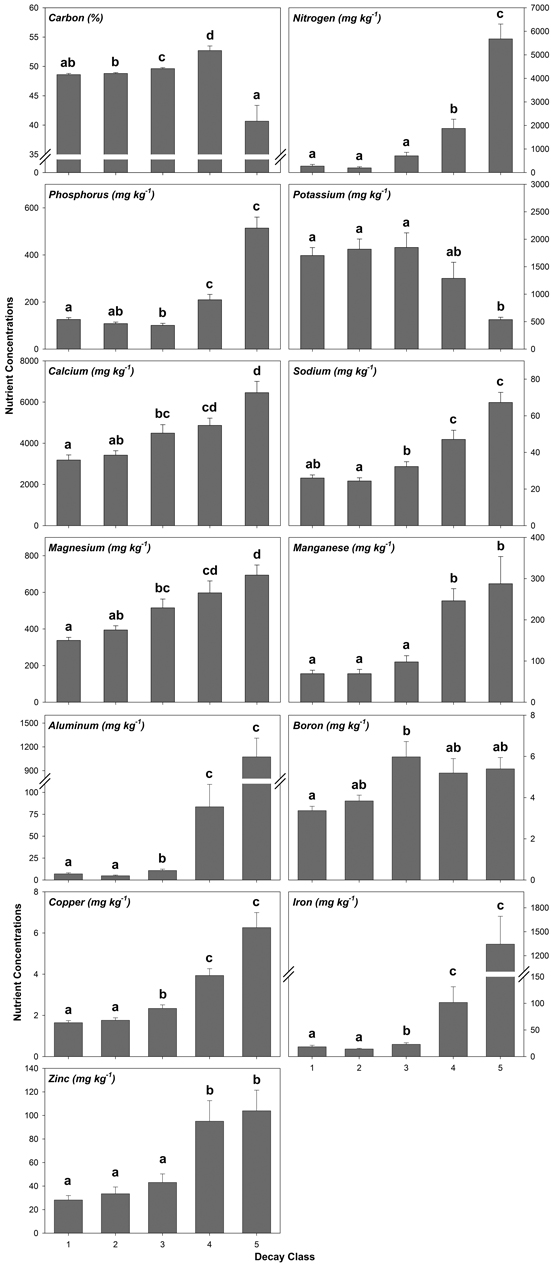
Fig. 2. Mean coarse woody debris (CWD) nutrient concentrations by decay class pooled across species/wood type. Values with similar letters are not significantly different (p > 0.05). Sample sizes within decay classes 1–3 are n = 50, decay class 4 are n = 20, and decay class 5 are n = 10.
4 Discussion
4.1 Observations and comparison to existing literature
We have presented extensive data on nutrient concentrations in woody debris of P. tremuloides forests in the upper Great Lakes. We note that FWD nutrient concentrations were significantly greater than those of CWD. Nutrient concentrations in both CWD and FWD differed among decay classes and species and showed numerous interactions. Nutrient concentrations tended to increase with decay class, most notably in CWD. These differences in nutrient concentrations among sizes, decay classes, and species of downed woody debris revealed in this study highlight the complex and variable influence that CWD and FWD can have on ecosystem nutrient stocks in P. tremuloides forests. This complexity and variability is frequently observed in other studies of woody debris, particularly CWD, conducted in other forest types (Miller 1983; Krankina et al. 1999; Laiho and Prescott 2004; Kuehne et al. 2008; Herrmann and Bauhus 2012; Gonzalez-Polo et al. 2013; Lombardi et al. 2013), and it reinforces the importance of examining these factors in various regions and across a range of forest conditions. These data provide an important baseline for increasing our knowledge of the role CWD and FWD play in forested ecosystems, specifically northern P. tremuloides forests. With this information, we can assess future productivity of intensively managed forests, particularly those slated for use as bioenergy feedstocks.
FWD nutrient concentrations from our study were generally consistent with those from other studies examining similar forest types and species (Sykes and Barr 1973; Johnston and Bartos 1977; Whittaker et al. 1979; Jokela et al. 1981; Lang et al. 1982; Miller 1983; Pastor and Bockheim 1984; Wang et al. 1995). This similarity was particularly true for decay class 1 samples, as nutrient concentrations of more-decayed FWD have been less studied (Miller 1983; Mladenoff et al. 2010). Given the limited research on nutrients in FWD (but see Johnston and Bartos 1977; Whittaker et al. 1979; Miller 1983), many of the sources we used for comparison contained values for live branches, while only a few were for downed wood. For CWD, nutrient concentrations in decay classes 1–4 were generally consistent with those reported in other studies of similar forest types and species for most of the nutrients examined (Sykes and Barr 1973; Lambert et al. 1980; Miller 1983; Alban and Pastor 1993; Krankina et al. 1999; Holub et al. 2001; Saunders et al. 2011). Additionally, our observed trends in nutrient concentrations for CWD through all decay classes were generally consistent with other studies that used a five-class decay system (Arthur and Fahey 1990; Means et al. 1992; Krankina et al. 1999).
4.2 Coarse woody debris vs. fine woody debris
Nutrient concentrations in FWD tended to be higher than those in CWD, which is consistent with the trends observed in other studies (Johnston and Bartos 1977; Whittaker et al. 1979; Miller 1983). This result is partly attributable to the high nutrient concentrations in branch bark (Lang et al. 1982) and the high bark-to-wood ratio (surface area) of FWD compared to CWD (Zavitkovski 1971). Additionally, our decay class 1 FWD samples generally came from material harvested the previous winter. Given this season of harvest, nutrients had been translocated from leaves and were stored in various parts of the tree, including the branches (Alban 1985). As trees were felled, the nutrient-rich branches became inputs of nutrient-rich FWD, likely containing greater nutrient concentrations than if harvested during the growing season.
Few studies have examined nutrient concentrations through various decay stages in FWD, particularly in the Great Lakes region (but see Miller 1983; Mladenoff et al. 2010). Our results indicated several nutrient concentrations in FWD increased and a few decreased as decay progressed. One criterion for defining our decay class 2 FWD samples was that bark was sloughing but still mostly present. For P. tremuloides, half of the nutrient-rich branch bark has been observed to slough off within four years, and macronutrient concentrations decrease by half between two to five years (Miller 1983). This finding would indicate that, despite the loss of some bark, our sampled material had not been on the ground for an extended period and was still in the early stages of decay. However, FWD commonly decays quickly (Moroni and Ryan 2010) relative to CWD due to its closer proximity to the ground (Söderström 1988) and higher relative surface area available for decomposing organisms (Kruys and Jonsson 1999).
We observed decreases in FWD K and P concentrations between decay classes 1 and 2, which are consistent with patterns observed for CWD in our study and by other studies of FWD and CWD (Grier 1978; Miller 1983). Potassium concentrations have been shown to decrease by half within approximately one year of decay in P. tremuloides FWD in northern Minnesota (Miller 1983). In our study, K concentrations decreased between 27–72% from decay class 1 to 2 within all species. This finding suggests that our decay class 2 FWD had been dead and on the ground for close to two years, which seems plausible given the presence of bark and the slightly longer rate of bark sloughing observed by Miller (1983). In addition, K is known to be highly mobile in woody debris, and concentrations tend to decrease before mass loss or fragmentation occurs (Krankina et al. 1999). Phosphorus tends not to be a limiting nutrient in temperate, terrestrial ecosystems and has been shown to leach from CWD over time (Harmon et al. 1986). Therefore, microbial decomposers are likely not limited by P, leading to higher rates of leaching of the unused P from CWD (Holub et al. 2001). This pattern for CWD may also apply to FWD leading to the lower levels of P we observed in decay class 2 materials.
Surprisingly low N values were observed in FWD and some CWD samples. Specifically, N in P. tremuloides and A. rubrum CWD was below the detectable limit of 24 mg kg–1 in decay classes 1 and 2 and decay class 1, respectively. Since woody debris samples were taken from both intact forest and clearcuts, many of the decay class 1 samples came from freshly harvested wood in the clearcut areas that were cut over the previous winter. Because N and other nutrients have been observed to increase in living twigs and stem material in the fall as a result of leaf abscission (Grigal et al. 1976; Alban 1985) and given that many of our decay class 1 FWD and decay class 1 and 2 CWD samples came from winter-harvested material, we would expect to see higher N concentrations in these samples. However, it is possible that some of our decay class 1 and 2 CWD came from dead standing material that was knocked over during harvest operations and would be older than previously live wood. Brais et al. (2006) noted an initial decline in N concentration after tree death followed by an exponential increase in concentration in boles of P. tremuloides Michx. However, N concentrations modeled by Brais et al. (2006) were still orders of magnitude greater than in our study despite this initial decline. Aside from this, we have no clear explanation to offer regarding the low observed N values.
4.3 Trends across decay classes
Many biotic factors influence nutrient concentrations in downed woody debris, most notably decomposing organisms. Specifically, deadwood-dependent insects break down material and create galleries for other organisms to invade (Harmon et al. 1986). Insect use of FWD can depend on diameter and substrate species (Jonsell et al. 2007). Fungi play a critical and complex role in woody debris nutrient cycling through sequestration and redistribution of crucial nutrients via their mycelia (Boddy and Watkinson 1995). Wood-decay fungal species richness is quite high in P. tremuloides forests, where FWD is a key substrate for these species (Brazee et al. 2012). Nitrogen-fixation by microbes can be an important source of N input into woody debris, particularly in slightly decayed and moist material (Roskoski 1980), and can vary with type of microbe, wood species, and seasonal interactions (Hendrickson 1991; Hicks et al. 2003). While less likely for FWD, CWD is known to be a key substrate for tree regeneration in later stages of decay (Cornett et al. 2001; Marx and Walters 2008; Bolton and D’Amato 2011), and even decay class 4 CWD showed penetration by plant roots, which would translocate nutrients into the wood (Harmon et al. 1986).
Abiotic factors can also influence woody debris nutrient concentrations. Nutrient input via litterfall and throughfall can be a significant source of nutrients for CWD, particularly in the moist, coastal forests of the Pacific Northwest (Grier 1978) but also in forests of the northeastern U. S. (Foster and Lang 1982). Litterfall and throughfall likely provided some level of nutrient input to our CWD samples, partially explaining the notable increases in nutrient concentrations of decay class 4 and 5 samples. Litterfall and throughfall may have provided nutrient inputs to FWD in our study, particularly larger pieces of FWD. However, this material has been shown to decompose rapidly relative to CWD (Moroni and Ryan 2010) and any inputs from these nutrient sources are likely minimal.
We observed no significant change in any CWD nutrient concentration from decay class 1 to 2, likely due to a lag period in decomposer colonization and establishment (Harmon et al. 1986). Factors that diminish accessibility to decomposers include large substrate diameter (Buchanan and Englerth 1940) and lack of ground contact (Söderström 1988). Since a number of our decay class 1 and 2 samples were collected from recently clearcut areas, it is possible that the open conditions allowed for rapid drying of CWD, resulting in slow decomposer colonization and activity. It is also possible that exposure to open conditions may have affected some nutrient concentrations in FWD between decay classes 1 and 2. Additionally, low N concentrations may slow the decay of wood (Merrill and Cowling 1965). Despite numerous other works documenting initial increases in N concentration in deadwood due to immobilization by decomposers (Lambert et al. 1980; Maser and Trappe 1984; Alban and Pastor 1993; Laiho and Prescott 2004), we did not observe this dynamic, suggesting there may be a temporal lag between mortality and noticeable changes in nutrient concentrations.
4.4 Trends among species
Trends among species in both FWD and CWD nutrient concentrations showed significant variation. Specifically, among FWD, B. papyrifera contained the highest nutrient concentrations and, among CWD, it contained relatively low nutrient concentrations. Both cases were likely attributable to the decay-resistant bark of this species. In the case of FWD, B. papyrifera contains a greater proportion of nutrient-rich bark compared to nutrient-poor bole wood (Jokela et al. 1981). In the case of CWD, the bark is in lesser proportion to bole wood and may provide a barrier in early decay stages dampening any initial changes to nutrient concentrations via biotic or abiotic avenues. F. nigra generally showed the highest CWD nutrient concentrations, whereas the lowest concentrations for both CWD and FWD were observed in A. rubrum. Boles of P. tremuloides contain high nutrient concentrations relative to other species (Alban and Pastor 1993), particularly Ca, an important element in P. tremuloides forests (Alban et al. 1978). In this study, concentrations of Ca were highest in P. tremuloides for both CWD and FWD.
4.5 Management implications and future research
Understanding the nutrient dynamics of woody debris is important for assessing site quality and productivity following disturbance, which typically increases the abundance of woody debris on a site. Harvests, specifically bioenergy feedstock harvests, directly manipulate the pools of CWD and FWD through removal or retention and can impact future growth (Helmisaari et al. 2011). Within P. tremuloides forests of the Great Lakes region, such harvests result in large inputs of FWD (Rittenhouse et al. 2012; Klockow et al. 2013). These studies indicate that season-of-harvest and species can play a major role in woody debris retention following bioenergy feedstock harvesting. Our results suggest that smaller FWD and highly decayed CWD can represent important sources of nutrients for these intensively managed ecosystems. Although less studied, micronutrients are necessary for tree physiological health and nutrition (Boardman and McGuire 1990; Stone 1990; Hagen-Thorn and Stjernquist 2005; Pallardy 2008) and, based on observations from this study, significant quantities of highly decayed woody debris could represent an important pool of these elements. Given the high nutrient concentrations in FWD observed in our study and rapid decomposition rates of this material, we expect that retaining significant stocks of this material could help mitigate short term nutrient losses due directly to harvest removals or indirectly from leaching following harvest. Additionally, active retention of CWD stocks could provide an important source of micronutrients as stands age.
In terms of species, A. rubrum consistently contained low nutrient concentrations in both CWD and FWD. In contrast, F. nigra CWD displayed high nutrient concentrations, suggesting that it may influence site nutrient availability especially given its prevalence in the studied forest type. Where site quality is low, focus should be placed on retention of nutrient-rich species including F. nigra and P. tremuloides. The recalcitrant bark of B. papyrifera likely strongly influences patterns of nutrient loss and accumulation in this species. Retention of B. papyrifera CWD and FWD could provide continued nutrient inputs to the forest floor and soil, as well as a source of habitat via larger diameter material, after other species have fully decomposed. In addition, retention of P. tremuloides CWD may contribute to longer term nutrient availability, notably Ca.
Future research on management in P. tremuloides forests could be guided by insight gleaned from the size-, species-, and decay-specific nutrient concentration data characterized in this study. Additionally, nutrient dynamics and modeling studies could further use this information toward detailed assessments of impacts on future habitat, nutrient stores, and productivity of a site following disturbance, especially within the context of intensive bioenergy feedstock harvesting.
Acknowledgements
We would like to thank Miranda Curzon, Chad Roy, and Josh Kragthorpe for assistance with sample collection and Mike Carson, John Segari, Robert Seavey, and Matt Dubay for assistance with sample processing. In addition, we thank the Research Analytical Laboratory at the University of Minnesota in St. Paul, Minnesota and the U.S. Forest Service Northern Research Station in Grand Rapids, Minnesota for nutrient analyses. We would like to express gratitude to the St. Louis County Land Department and the Minnesota Department of Natural Resources for providing field sites as well as administrative and technical support. This research was supported by the Minnesota Forest Resources Council and USDA/DOE Biomass Research Development Initiative. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
References
Alban D.H. (1985). Seasonal changes in nutrient concentration and content of aspen suckers in Minnesota. Forest Science 31(3): 785–794.
Alban D.H., J. Pastor. (1993). Decomposition of aspen, spruce, and pine boles on two sites in Minnesota. Canadian Journal of Forest Research 23: 1744–1749. http://dx.doi.org/10.1139/x93-220.
Alban D.H., D.A. Perala, B.E. Schlaegel. (1978). Biomass and nutrient distribution in aspen, pine, and spruce stands on the same soil type in Minnesota. Canadian Journal of Forest Research 8: 290–299. http://dx.doi.org/10.1139/x78-044.
Arthur M.A., T.J. Fahey. (1990). Mass and nutrient content of decaying boles in an Engelmann spruce-subalpine fir forest, Rocky Mountain National Park, Colorado. Canadian Journal of Forest Research 20: 730–737. http://dx.doi.org/10.1139/x90-096.
Åström M., M. Dynesius, K. Hylander, C. Nilsson. (2005). Effects of slash harvest on bryophytes and vascular plants in southern boreal forest clear-cuts. Journal of Applied Ecology 42(6): 1194–1202. http://dx.doi.org/10.1111/j.1365-2664.2005.01087.x.
Boardman R., D.O. McGuire. (1990). The role of zinc in forestry. I. Zinc in forest ecosystems, environments and tree nutrition. Forest Ecology and Management 37: 167–205. http://dx.doi.org/10.1016/0378-1127(90)90054-F.
Boddy L., S.C. Watkinson. (1995). Wood decomposition, higher fungi, and their role in nutrient redistribution. Canadian Journal of Botany 73(Suppl. 1): S1377–S1383. http://dx.doi.org/10.1139/b95-400.
Bolton N.W., A.W. D’Amato. (2011). Regeneration responses to gap size and coarse woody debris within natural disturbance-based silvicultural systems in northeastern Minnesota, U.S.A. Forest Ecology and Management 262: 1215–1222. http://dx.doi.org/10.1016/j.foreco.2011.06.019.
Brais S., D. Paré, C. Lierman. (2006). Tree bole mineralization rates of four species of the Canadian eastern boreal forest: implications for nutrient dynamics following stand-replacing disturbances. Canadian Journal of Forest Research 36: 2331–2340. http://dx.doi.org/10.1139/x06-136.
Brazee N.J., D.L. Lindner, S. Fraver, A.W. D’Amato, A.M. Milo. (2012). Wood-inhabiting, polyporoid fungi in aspen-dominated forests managed for biomass in the U.S. Lake States. Fungal Ecology 5(5): 600–609. http://dx.doi.org/10.1016/j.funeco.2012.03.002.
Brown J.K. (1974). Handbook for inventorying downed woody material. U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station, General Technical Report INT-16. 24 p.
Buchanan T.S., G.H. Englerth. (1940). Decay and other volume losses in windthrown timber on the Olympic Peninsula, Washington. USDA Technical Bulletin 733. U.S. Government Printing Office, Washington, D.C.
Bunnell F.L., I. Houde. (2010). Down wood and biodiversity – implications to forest practices. Environmental Reviews 18: 397–421. http://dx.doi.org/10.1139/A10-019.
Conover W.J., R.L. Iman. (1981). Rank transformations as a bridge between parametric and nonparametric statistics. The American Statistician 35(3): 124–129.
Cornett M.W., K.J. Puettmann, L.E. Frelich, P.B. Reich. (2001). Comparing the importance of seedbed and canopy type in the restoration of upland Thuja occidentalis forests of northeastern Minnesota. Restoration Ecology 9(4): 386–396. http://dx.doi.org/10.1046/j.1526-100X.2001.94008.x.
Dynesius M., M. Åström, C. Nilsson. (2008). Microclimatic buffering by logging residues and forest edges reduces clear-cutting impacts on forest bryophytes. Applied Vegetation Science 11: 345–354. http://dx.doi.org/10.3170/2008-7-18457.
Foster J.R., G.E. Lang. (1982). Decomposition of red spruce and balsam fir boles in the White Mountains of New Hampshire. Canadian Journal of Forest Research 12: 617–626. http://dx.doi.org/10.1139/x82-094.
Gonzalez-Polo M., A. Fernández-Souto, A.T. Austin. (2013). Coarse woody debris stimulates soil enzymatic activity and litter decomposition in an old-growth temperate forest of Patagonia, Argentina. Ecosystems. http://dx.doi.org/10.1007/s10021-013-9665-0.
Grier C.C. (1978). Tsuga heterophylla-Picea sitchensis ecosystem of coastal Oregon: decomposition and nutrient balances of fallen logs. Canadian Journal of Forest Research 8(2): 198–206. http://dx.doi.org/10.1139/x78-031.
Grigal D.F., L.F. Ohmann, R.B. Brander. (1976). Seasonal dynamics of tall shrubs in northeastern Minnesota: biomass and nutrient element changes. Forest Science 22: 195–208.
Hagen-Thorn A., I. Stjernquist. (2005). Micronutrient levels in some temperate European tree species: a comparative field study. Trees 19: 572–579. http://dx.doi.org/10.1007/s00468-005-0416-5.
Harmon M.E., J.F. Franklin, F.J. Swanson, P. Sollins, S.V. Gregory, J.D. Lattin, N.H. Anderson, S.P. Cline, N.G. Aumen, J.R. Sedell, G.W. Lienkaemper, K. Cromack, Jr., K.W. Cummins. (1986). Ecology of coarse woody debris in temperate ecosystems. Advances in Ecological Research 15: 133–302. http://dx.doi.org/10.1016/S0065-2504(08)60121-X.
Hautala H., J. Jalonen, S. Laaka-Lindberg, I. Vanha-Majamaa. (2004). Impacts of retention felling on coarse woody debris (CWD) in mature boreal spruce forests in Finland. Biodiversity and Conservation 13: 1541–1554. http://dx.doi.org/10.1023/B:BIOC.0000021327.43783.a9.
Hendrickson O.Q. (1991). Abundance and activity of N2-fixing bacteria in decaying wood. Canadian Journal of Forest Research 21(9): 1299–1304. http://dx.doi.org/10.1139/x91-183.
Helmisaari H-S., K.H. Hanssen, S. Jacobson, M. Kukkola, J. Luiro, A. Saarsalmi, P. Tamminen, B. Tveite. (2011). Logging residue removal after thinning in Nordic boreal forests: long-term impact on tree growth. Forest Ecology and Management 261: 1919–1927. http://dx.doi.org/10.1016/j.foreco.2011.02.015.
Herrmann S., J. Bauhus. (2012). Effects of moisture, temperature, and decay stage on respirational carbon loss from coarse woody debris (CWD) of important European tree species. Scandinavian Journal of Forest Research 28(4): 346–357. http://dx.doi.org/10.1080/02827581.2012.747622.
Herrmann S., C.E. Prescott. (2008). Mass loss and nutrient dynamics of coarse woody debris in three Rocky Mountain coniferous forests: 21 year results. Canadian Journal of Forest Research 38(1): 125–132. http://dx.doi.org/10.1139/X07-144.
Hicks W.T., M.E. Harmon, R.P. Griffiths. (2003). Abiotic controls on nitrogen fixation and respiration in selected woody debris from the Pacific Northwest, U.S.A. Ecoscience 10(1): 66–73.
Holub S.M., J.D.H. Spears, K. Lajtha. (2001). A reanalysis of nutrient dynamics in coniferous coarse woody debris. Canadian Journal of Forest Research 31(11): 1894–1902. http://dx.doi.org/10.1139/x01-125.
Johnston R.S., D.L. Bartos. (1977). Summary of nutrient and biomass data from two aspen sites in western United States. USDA Forest Service Research Note INT-227. Intermountain Forest and Range Experiment Station, Ogden, Utah. 15 p.
Jokela E.J., C.A. Shannon, E.H. White. (1981). Biomass and nutrient equations for mature Betula papyrifera Marsh. Canadian Journal of Forest Research 11(2): 298–304. http://dx.doi.org/10.1139/x81-040.
Jonsell M., J. Hansson, L. Wedmo. (2007). Diversity of saproxylic beetle species in logging residues in Sweden – comparisons between tree species and diameters. Biological Conservation 138(1–2): 89–99. http://dx.doi.org/10.1016/j.biocon.2007.04.003.
Juutilainen K., P. Halme, H. Kotiranta, M. Mönkkönen. (2011). Size matters in studies of dead wood and wood-inhabiting fungi. Fungal Ecology 4: 342–349. http://dx.doi.org/10.1016/j.funeco.2011.05.004.
Klockow P.A., A.W. D’Amato, J.B. Bradford. (2013). Impacts of post-harvest slash and live-tree retention on biomass and nutrient stocks in Populus tremuloides Michx.-dominated forests, northern Minnesota, USA. Forest Ecology and Management 291: 278–288. http://dx.doi.org/10.1016/j.foreco.2012.11.001.
Krankina O.N., M.E. Harmon, A.V. Griazkin. (1999). Nutrient stores and dynamics of woody detritus in a boreal forest: modeling potential implications at the stand level. Canadian Journal of Forest Research 29(1): 20–32. http://dx.doi.org/10.1139/x98-162.
Kruys N., B.G. Jonsson. (1999). Fine woody debris is important for species richness on logs in managed boreal spruce forests of northern Sweden. Canadian Journal of Forest Research 29: 1295–1299. http://dx.doi.org/10.1139/x99-106.
Krzyszowska-Waitkus A., G.F. Vance, C.M. Preston. (2006). Influence of coarse wood and fine litter on forest organic matter composition. Canadian Journal of Soil Science 86(1): 35–46. http://dx.doi.org/10.4141/S05-040.
Kuehne C., C. Donath, S.I. Müller-Using, N. Bartsch. (2008). Nutrient fluxes via leaching from coarse woody debris in a Fagus sylvatica forest in the Solling Mountains, Germany. Canadian Journal of Forest Research 38: 2405–2413. http://dx.doi.org/10.1139/X08-088.
Laiho R., C.E. Prescott. (2004). Decay and nutrient dynamics of coarse woody debris in northern coniferous forests: a synthesis. Canadian Journal of Forest Research 34(4): 763–777. http://dx.doi.org/10.1139/x03-241.
Lambert R.L., G.E. Lang, W.A. Reiners. (1980). Loss of mass and chemical change in decaying boles of a subalpine balsam fir forest. Ecology 61(6): 1460–1473. http://dx.doi.org/10.2307/1939054.
Lang G.E., W.A. Reiners, G.A. Shellito. (1982). Tissue chemistry of Abies balsamea and Betula papyrifera var. cordifolia from subalpine forests of northeastern United States. Canadian Journal of Forest Research 12(2): 311–318. http://dx.doi.org/10.1139/x82-045.
Lombardi F., P. Cherubini, R. Tognetti, C. Cocozza, B. Lasserre, M. Marchetti. (2013). Investigating biochemical processes to assess deadwood decay of beech and silver fir in Mediterranean mountain forests. Annals of Forest Science 70: 101–111. http://dx.doi.org/10.1007/s13595-012-0230-3.
Marx L., M.B. Walters. (2008). Survival of tree seedlings on different species of decaying wood maintains tree distribution in Michigan hemlock-hardwood forests. Journal of Ecology 96: 505–513. http://dx.doi.org/10.1111/j.1365-2745.2008.01360.x.
Maser G., J.M. Trappe. (1984). The seen and unseen world of the fallen tree. USDA Forest Service, Pacific Northwest Forest Experiment Station, General Technical Report PNW-GTR-164. 56 p.
Means J.E., P.C. MacMillan, K. Cromack, Jr. (1992). Biomass and nutrient content of Douglas-fir logs and other detrital pools in an old-growth forest, Oregon, U.S.A. Canadian Journal of Forest Research 22(10): 1536–1546. http://dx.doi.org/10.1139/x92-204.
Merrill W., E.B. Cowling. (1965). Effect of variation in nitrogen content of wood on rate of decay. Phytopathology 55: 1067–1068.
Miller W.E. (1983). Decomposition rates of aspen bole and branch litter. Forest Science 29(2): 351–356.
Mladenoff D.J, J.A. Forrester, J. Schatz. (2010). Impacts of biomass removal on carbon and nutrient pools in Wisconsin northern hardwood forests: establishment of a long-term study. Final Report on the Environmental and Economic Research and Development Program, Public Service Commission of Wisconsin and The Statewide Energy Efficiency and Renewables Administration. 38 p.
Moroni M.T., D.A.J. Ryan. (2010). Deadwood abundance in recently harvested and old Nova Scotia hardwood forests. Forestry 83(2): 219–227. http://dx.doi.org/10.1093/forestry/cpq007.
Pallardy S.G. (2008). Physiology of woody plants. 3rd edition. Elsevier, Amsterdam–Boston. 464 p.
Pastor J., J.G. Bockheim (1984). Distribution and cycling of nutrients in an aspen – mixed-hardwood-spodosol ecosystem in northern Wisconsin. Ecology 65(2): 339–353. http://dx.doi.org/10.2307/1941398.
Rittenhouse T.A.G., D.M. MacFarland, K.J. Martin, T.R. Van Deelen. (2012). Downed wood associated with roundwood harvest, whole-tree harvest, and unharvested stands of aspen in Wisconsin. Forest Ecology and Management 266: 239–245. http://dx.doi.org/10.1016/j.foreco.2011.11.029.
Roskoski J.P. (1980). Nitrogen fixation in hardwood forests of the northeastern United States. Plant and Soil 54(1): 33–44. http://dx.doi.org/10.1007/BF02181997.
Saunders M.R., S. Fraver, R.G. Wagner. (2011). Nutrient concentration of down woody debris in mixedwood forests in central Maine, USA. Silva Fennica 45(2): 197–210.
Siitonen J. (2001). Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecological Bulletins 49: 11–41.
Söderström L. (1988). Sequence of bryophytes and lichens in relation to substrate variables of decaying coniferous wood in northern Sweden. Nordic Journal of Botany 8(1): 89–97. http://dx.doi.org/10.1111/j.1756-1051.1988.tb01709.x.
Sollins P. (1982). Input and decay of coarse woody debris in coniferous stands in western Oregon and Washington. Canadian Journal of Forest Research 12(1): 18–28. http://dx.doi.org/10.1139/x82-003.
Stone E.L. (1990). Boron deficiency and excess in forest trees: a review. Forest Ecology and Management 37: 49–75. http://dx.doi.org/10.1016/0378-1127(90)90046-E.
Sykes J.M., C.J. Barr. (1973). Dry weight and mineral composition estimates for 15-year-old mixed hardwood coppice in Roudsea Wood. Merlewood Research and Development Paper 50. Merlewood, U.K. 18 p.
Wang J.R., A.L. Zhong, P. Comeau, M. Tsze, J.P. Kimmins. (1995). Aboveground biomass and nutrient accumulation in an age sequence of aspen (Populus tremuloides) stands in the Boreal White and Black Spruce Zone, British Columbia. Forest Ecology and Management 78(1–3): 127–138. http://dx.doi.org/10.1016/0378-1127(95)03590-0.
Whittaker R.H., G.E. Likens, F.H. Bormann, J.S. Eaton, T.G. Siccama. (1979). The Hubbard Brook ecosystem study: forest nutrient cycling and element behavior. Ecology 60(1): 203–220. http://dx.doi.org/10.2307/1936481.
Zavitkovski J. (1971). Dry weight and leaf area of aspen trees in northern Wisconsin. In: Forest biomass studies. University of Maine, Orono, Life Science and Agricultural Experiment Station Miscellaneous Publication 132. p. 193–205.
Total of 62 references
Appendix
The appendix is viewable in a new window/tab. It contains five tables with data on:
- Pairwise comparisons of species for all nutrients in fine woody debris (FWD) (Table A1).
- Mean coarse woody debris (CWD) nutrient concentrations for each species by decay class (Table A2).
- Analysis of variance (ANOVA) table with pairwise comparisons for all macronutrients (C, N, P, K, Ca, and Mg) in coarse woody debris (CWD) by species and decay class (Table A3).
- Analysis of variance (ANOVA) table with pairwise comparisons for all micronutrients (Na, Mn, Al, B, Cu, Fe, and Zn) in coarse woody debris (CWD) by species and decay class (Table A4).
- Mean coarse woody debris (CWD) nutrient concentrations for each decay class pooled across species/wood type (Table A5).

