Continuous picking may increase bilberry yields
Manninen O., Peltola R. (2019). Continuous picking may increase bilberry yields. Silva Fennica vol. 53 no. 3 article id 10043. https://doi.org/10.14214/sf.10043
Highlights
- Bilberry fruit production and fruit set increased under continuous picking by rake in three-year study
- Bilberry flower number and fruit mass were not affected by picking
- Bilberry compensated for biomass loss
- The highest relative deciduous species abundance was found in the picking treatment plots at the end of the experiment.
Abstract
Accumulated knowledge about the health benefits of bilberry (Vaccinium myrtillus L.) has increased the demand and utilization of wild bilberries. Intensive berry picking by metal rakes is believed to cause damage in bilberry stands in areas under continuous picking pressure, and hence expected to hamper the production of berries in forthcoming years. We conducted an experiment to examine the effect of continuous bilberry picking by metal rake on the number of bilberry flowers and fruits, fruit mass, compensation for biomass loss after picking, and plant functional type abundance in the understorey in northern Finland. Bilberry lost less than 0.5% of its biomass annually during the three-year study period due to rake harvesting. The number of flowers was not significantly affected by damage caused by picking, while both fruit production and fruit set increased without any indication of reduced fruit mass, and biomass loss was fully compensated. Moreover, the relative abundance of plant functional types was not affected by picking during the study. We suggest that the low intensity and timing of damage act as a buffer against the adverse effects of picking on bilberry fruit production. On the basis of this study, it is reasonable to anticipate that there are no indications that current intensive berry picking would not be on a sustainable level.
Keywords
sustainability;
Vaccinium myrtillus;
wild forest berries;
collectable goods
-
Manninen,
Natural Resources Institute Finland (Luke), Bioeconomy and environment, Paavo Havaksentie 3, FI-90014 University of Oulu, Finland
E-mail
outi.manninen@luke.fi

- Peltola, Natural Resources Institute Finland (Luke), Bioeconomy and environment, Ounasjoentie 6, FI-96200 Rovaniemi, Finland E-mail rainer.peltola@luke.fi
Received 6 September 2018 Accepted 4 September 2019 Published 6 September 2019
Views 60562
Available at https://doi.org/10.14214/sf.10043 | Download PDF

1 Introduction
Bilberry (Vaccinium myrtillus L.) is a wild growing, deciduous dwarf shrub commonly found throughout Eurasia and western parts of North America. Bilberry expands mainly by effective rhizomatous growth regardless of luxurious flowering and an abundant production of berries. Both the berries and leaves of bilberry have been utilized as food and folk medicine for centuries in Nordic countries. Enhancing knowledge about the health benefits of bilberry (Riihinen et al. 2008) and their applications in cosmetics and well-being products have increased the demand for bilberry. As a response to the increasing demand, the utilization of wild yields of bilberry is continuously increasing.
The general concerns of the utilization of wild bilberry yields are linked to assumed negative effects of intensive picking to subsequent berry production. This concern rests mainly on the harvesting practices, i.e. the intensive use of metal rakes in bilberry picking, which is claimed to cause visible damage in vegetation, and hence is expected to hamper the production of berries in forthcoming years. Bilberry fruit production is, indeed, a very uncertain process. For instance, forest management, history and stand age affect the abundance (Hedwall et al. 2013; Eldegard et al. 2019) and fruit production of bilberry (Nielsen et al. 2007; Miina et al. 2009). Moreover, the fruit production of bilberry is dependent on numerous climate-related factors (Selås et al. 2015; Boulanger-Lapointe et al. 2017), leading to a high between-year variation in bilberry yields. Intensive berry picking by rakes may cause damage by ripping and removing the biomass of bilberry comparable to herbivory. Natural herbivory induces plant chemical defense responses (Koo and Howe 2009), and, hence, is not totally analogous to biomass loss by commercial berry picking, despite similar mechanical damages (Seldal et al. 2016). However, bilberry fruit production has been found to be reduced both under natural herbivory (Fernández-Calvo and Obeso 2004; Hegland et al. 2005, 2010), as well as in studies mimicking herbivory (Tolvanen et al. 1993b), and after clipping (Stark et al. 2010; Nestby et al. 2014) due to the allocation of resources to increased vegetative growth. Long-term vegetation changes towards more fast-growing graminoids in vegetation after biomass loss by clipping may lead to a lower sexual reproduction of bilberry (Mathisen et al. 2010) and bilberry yields.
Picking by plastic hand rake, commonly used in household and leisure picking, or picking by long-handed metal rake applied for more intensive purposes, did not diminish the bilberry fruit production in the following two years even when raking was applied intentionally by force (Manninen and Peltola 2013). However, in the study by Manninen and Peltola (2013), the picking treatment was applied only once which may not correspond to a practical utilization of wild berry yields, where the same areas may be under continuous picking pressure. In order to fill this gap in knowledge, we conducted an experiment to examine the effect of continuous bilberry picking on subsequent berry production. A long-handed metal rake was applied in the picking treatment to mimic an intensive bilberry picking event over the same areas during three years. To our knowledge, this is the first experimental study to investigate bilberry production and vegetation responses to continuous picking pressure. The research questions were: 1) Is the bilberry fruit production and the quality of berries reduced by continuous picking? 2) Do the fast-growing graminoids increase at the expense of other plant functional types in vegetation after continuous picking pressure? Answering these questions gives novel knowledge on a sustainability of current bilberry picking practice in areas under continuous picking pressure.
2 Materials and methods
2.1 Study sites
The experiment was conducted in a northern boreal forest area (66°32´N, 25°00´E) in Finland during 2012–2015. The meteorological data of the experimental area is shown in Table 1. Four sites, rich in berry-producing bilberry plants, were chosen for the experiment. The sites were located in an area of approximately one square km. Common understorey species at the sites together with bilberry are lingonberry (V. vitis-idaea L.) and wavy hair-grass (Deschampsia flexuosa [L.] Trin.). A thick layer of bryophytes, consisting of species such as Pleurozium scheberi (Brid.) Mitt. and Hylocomium splendens (Hedw.) Schimp., are commonly found in the forest floor.
| Table 1. Duration of thermal growing season (d), effective temperature sum of growing season (°Cd), annual mean temperature (°C) and annual mean precipitation (mm) in the study area in 2012–2015. | ||||
| Year | Duration of thermal growing season (d) ab | Effective temperature sum of growing season (°Cd) b | Annual mean temperature (°C) c | Annual mean precipitation (mm) c |
| 2012 | 156 | 921 | 0.7 | 692 |
| 2013 | 157 | 1246 | 2.2 | 481 |
| 2014 | 136 | 1141 | 2.8 | 515 |
| 2015 | 151 | 903 | 2.7 | 783 |
| a Number of days when the mean daily temperature exceeds 5 °C, b Based on data collected by the nearest weather station at Rovaniemi airport (Finnish Meteorological Institute), c Based on grid data (Finnish Meteorological Institute). | ||||
2.2 Experimental design
Sixty permanent study plots (1 × 1 m) were established in the experimental area in August 2012 (Table 2). From these 60 study plots, 20 study plots were located at site 1, 10 study plots at site 2, 10 study plots at site 3, and 20 study plots at site 4. The study plots were randomly assigned to the control and picking treatments within each site. The number of replicates was 10, 5, 5 and 10 at sites 1, 2, 3 and 4, respectively. Study plots at each site were established at least 5 meters apart from each other.
| Table 2. Numbers of all study plots, control and picking treatment plots at four sites during the study. Measurements of the bilberry and aboveground vegetation in the control and picking treatment plots during the study are denoted by plus and minus -signs for each year separately. | ||||
| Number of study plots and the measurements in control (C) and picking treatment plots (PT) | 2012 | 2013 | 2014 | 2015 |
| Number of | ||||
| all study plots, sum (at sites 1,2,3,4) | 60 (20,10,10,20) | 59 (20,9*,10,20) | 59 (20,9,10,20) | 40 (10,0,10,20) |
| control plots, sum (at sites 1,2,3,4) | 30 (10,5,5,10) | 29 (10,4*,5,10) | 29 (10,4,5,10) | 20 (5,0,5,10) |
| treatment plots, sum (at sites 1,2,3,4) | 30 (10,5,5,10) | 30 (10,5,5,10) | 30 (10,5,5,10) | 20 (5,0,5,10) |
| Number of berries, C/PT | +/+ | +/+ | +/+ | +/+ |
| Weight of berries, C/PT | -/+ | -/+ | -/+ | +/+ |
| Biomass removed by raking, C/PT | -/+ | -/+ | -/+ | -/- |
| Aboveground undamaged biomass** | + | - | - | - |
| Number of flowers, C/PT | -/- | +/+ | +/+ | -/- |
| Abundances of vascular plant, moss, litter and soil C/PT | -/- | +/+ | +/+ | +/+ |
| Aboveground biomass*** | -/- | -/- | -/- | +/+ |
| * One control plot was lost due to the tree felling during the summer, time between flowering and berry maturation, at the site 2. C/PT = Control/Picking treatment, + = measured, - = not measured, ** aboveground biomass of bilberry collected outside the study plots, *** aboveground biomass of all plant species collected from the study plots at the end of the experiment. | ||||
In 2012, the number of berries was counted in the control plots without picking them, and the first raking treatment was applied in the picking treatment plots. The picking treatment was applied by raking vegetation, mimicking intense picking for commercial purposes, using a long-handed metal rake. The width of the rake was 20 cm having evenly distributed, round (diameter 2.5 mm) metal tines, located 0.5 cm apart. Vegetation and the moss layer in the plots were carefully examined by hand after raking to ensure that all berries were collected in the picking treatment plots. Berries, which were picked in the picking treatment plots, were counted, air dried for two hours, pooled and weighed. The biomass, which was ripped from the vegetation by raking, was collected and the biomass of bilberry was separated from other plant species’ biomass, dried at 80 °C for 24 hours, and weighed. Undamaged, aboveground biomass of bilberry was measured by cutting randomly selected biomass samples (50 × 50 cm) outside the study plots at each site (N = 6 at each site) in 2012. The biomass of undamaged bilberry was treated similarly to the ripped biomass by raking in the picking treatment.
The number of bilberry flowers was counted in the control and picking treatment plots in 2013 and 2014. Bilberry flower counting is a very time-consuming work, which has to be accomplished within a short time period between flower development and wilting. Therefore the bilberry flowers were counted only in half of the control and picking treatment plots in 2013 (15 controls and 15 treatment plots, altogether 30 out of 60 study plots).
The number of berries was counted in the control plots in 2013 and 2014 as in 2012 (Table 2). Raking treatments were applied in the picking treatment plots, and the collected berries and the biomass which were removed by picking in 2013 and 2014 were processed as in 2012.
In 2015, all berries were picked by hand in the control and picking treatment plots. Raking was not applied in the picking treatment plots in 2015, due to the fact that damage caused by raking at that point would not likely affect the berry production in the same year. The collected berries in 2015 were treated as in 2012.
Plant abundances were estimated using the point frequency method at 100 systematically placed sampling points on each plot in July during 2013–2015. All the hits of each species were recorded and their abundances were pooled into plant functional types (deciduous dwarf shrubs, evergreen dwarf shrubs, graminoids and forbs) or total vegetation level. Mosses, litter and bare soil were recorded only once per hit, and, thus, their abundance corresponds to their cover in the ground layer.
In 2015, the aboveground biomass at randomly selected control (N = 6) and picking treatment (N = 6) plots was collected by cutting, and the biomass of bilberry and graminoid species was separated from other species, dried at 80 °C for 24 hours, and weighed.
One control plot was lost due to a felled tree after the flowering period at site 2, decreasing the number of study plots from the original 60 to 59 in 2013 (Table 2). Moreover, a timber harvester passed across the area and destroyed 19 study plots (10 at the site 1 and 9 at the site 2) in 2015. The number of study plots was 40, from which 20 were control plots and 20 picking treatment plots, at the end of the experiment in 2015.
The bilberry biomass, which was ripped by raking in the picking treatment during the experiment, and the average site-specific undamaged biomass of bilberry in 2012 are shown in Table 3.
| Table 3. Undamaged aboveground biomass of bilberry (Vaccinium myrtillus) (dry weight g m–2, mean ± SE) in 2012 and the amount of biomass, which was removed by rake together with berries in the berry picking treatment plots at four study sites during 2012–2014. The proportion of removed biomass from undamaged bilberry biomass is shown for 2012. | ||||||
| Site | 2012 | 2013 | 2014 | |||
| Undamaged biomass (g m–2)* | Removed biomass (g m–2) | Proportion of removed biomass from total biomass (%) | Removed biomass (g m–2) | Removed biomass (g m–2) | ||
| 1 | 145.28 ± 16.21 | 0.59 ± 0.15 | 0.40 | 0.31 ± 0.08 | 2.63 ± 1.10 | |
| 2 | 127.90 ± 26.66 | 0.19 ± 0.10 | 0.15 | 0.88 ± 0.39 | 0.59 ± 0.19 | |
| 3 | 196.57 ± 21.89 | 0.28 ± 0.10 | 0.14 | 0.74 ± 0.22 | 0.43 ± 0.13 | |
| 4 | 153.55 ± 16.10 | 0.63 ± 0.14 | 0.41 | 0.35 ± 0.10 | 0.69 ± 0.25 | |
| * Biomass of bilberry collected at randomly selected 50 × 50 cm plots outside the study plots at each site (N = 6). | ||||||
2.3 Statistical analysis
The initial difference in the number of berries between the control and picking treatment plots in 2012 was tested by using a one-factor ANOVA.
A linear mixed model was used to test the effect of picking treatment on the number of berries and flowers, fruit set, total vascular plant abundance, the relative abundance of plant functional types, and the relative abundance of mosses, litter and bare soil between the years. The fruit set of bilberry was estimated as the percentage value (number of mature berries/number of flowers × 100) per treatment. The relative abundance of plant functional types (deciduous dwarf shrubs, evergreen dwarf shrubs, graminoids and forbs) was calculated as the percentage values of total vascular plant abundance. Bilberry comprises 91.7% and bog whortleberry (V. uliginosum L.) 8.3% of the deciduous species abundance in the study. In the ground layer, the relative abundance of mosses, litter and bare soil was calculated as the percentage value from the total ground layer abundance.
The full model included the picking treatment as a fixed effect, plots nested within site as a random effect, and year as a repeated effect in the analysis testing the effect of picking on the number of berries, flowers, fruit set, the total vascular plant abundance, and the relative abundance of plant functional types. The best final model was selected by a backward eliminating method by removing the non-significant effects at the level p < 0.05 from the full model and by comparison of AIC values.
The variance estimates between sites and plots were almost zero which suggested removing the random effect from the model and to treat the site as a fixed effect. However, the site was not significant at the significance level p < 0.05 and was eliminated from the model. The best final model included picking treatment as a fixed effect, year as a repeated effect and their interactions in the analysis. Hence, the best final model was practically similar to a repeated measures ANOVA. However, linear mixed models were used due to the unbalanced data and missing values. Only the results of the best final model are reported. A compound symmetry covariance structure for year was used when testing the effect of picking on the number of berries, flowers, fruit set, and the relative abundance of plant functional types and mosses, litter and bare soil. An unstructured covariance structure was used in testing the effect of picking on the total plant abundance. An LSD post hoc-test at a significance level p < 0.05 was used to detect significant differences between years.
A one-factor ANOVA was used to test the difference between the mean fresh weight of berries in the control and picking treatment in 2015. The mean fresh weight of berries was derived by dividing the pooled fresh weight of berries in the control and picking treatment plots by their number in 2015.
The biomass of bilberry, pooled biomass of graminoids and pooled biomass of other vascular plant species collected in the control and picking treatment plots in 2015 was tested by a one-factor ANOVA.
Data was checked for the normality and homogeneity of variances, and log- and arcsine- transformed data was used in the analysis if the data did not fulfill the requirements. All statistical analyses were conducted using SPSS 25 for Windows (IBM Corp. 2017).
3 Results
The number of berries did not differ in the control and picking treatment plots prior to the experiment in 2012 (one-factor ANOVA: F(1,58) = 3.071, p = 0.085). The number of berries was significantly higher in the picking treatment plots than in the control plots in 2013–2015: the increment in berry number was approximately 79%, 65% and 56% in picking treatment plots compared to the controls in 2013, 2014 and 2015, respectively (Table 4) (Fig. 1). Between-year variation was not detected in the berry number in 2013–2015 (Table 4) (Fig. 1).
| Table 4. Results of linear mixed model analysis of the number of Vaccinium myrtillus berries, flowers and fruit set. | ||||||
| Source | Number of berries | Number of flowers | Fruit set | |||
| F | p | F | p | F | p | |
| Picking treatment (PT) | 7.92953,314 | 0.007 | 0.66528 | 0.422 | 6.86027,857 | 0.014 |
| Year (Y) | 0.22494,384 | 0.799 | 0.79728 | 0.380 | 0.36127,338 | 0.553 |
| PT × Y | 0.00894,384 | 0.992 | 0.23528 | 0.632 | 1.10527,388 | 0.302 |
| Numerator degrees of freedom Ndf = 1, 2, 2 for PT, Y and PT × Y in the number of berries, respectively. Ndf = 1, 1, 1 for PT, Y and PT × Y in the number of flowers and fruit set. Subscripts under F ratios indicate denominator degrees of freedom (Ddf). | ||||||
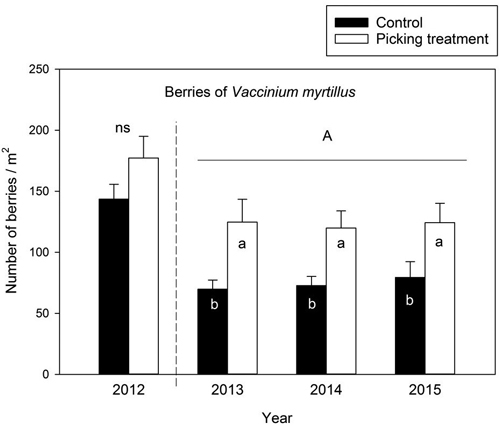
Fig. 1. Number of Vaccinium myrtillus berries (mean ± se) in the control and picking treatment plots before the experiment in 2012 and during the study period. The difference in the number of berries between the control and picking treatment plots in 2012 was not statistically significant (ns: not significant) as revealed by a one-factor ANOVA. The capital letter (A) above the columns denotes non-significant between-year variation in the number of berries during 2013–2015 (as revealed by a linear mixed model analysis). Small letters indicate the difference between the control and picking treatment at the level p < 0.05. In 2012: control N = 30, picking treatment N = 30. In 2013 and 2014: control N = 29, picking treatment N = 30. In 2015: control N = 20, picking treatment N = 20.
The number of flowers did not differ between the control and picking treatment plots in 2013 and 2014 (Table 4). The number of flowers was 142.93 ± 11.21 and 173.09 ± 28.04 flowers/m2 (mean ± SE) in the control and picking treatment plots in 2013, and 168.13 ± 18.33 and 180.53 ± 27.98 in 2014. The fruit set (%) of bilberry was higher in the picking treatment compared to the control in 2013 and 2014 (Table 4) (Fig. 2).
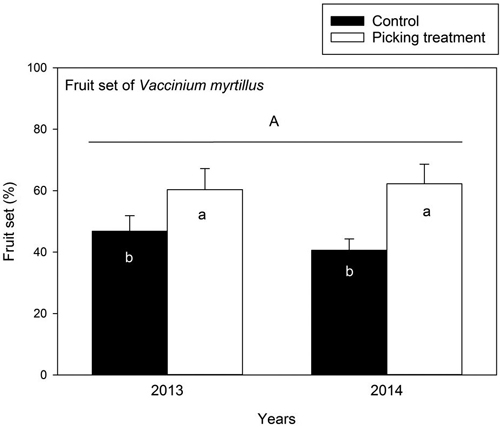
Fig. 2. Fruit set (%) of Vaccinium myrtillus (mean ± se) in control and picking treatment plots in 2013 and 2014. The fruit set was estimated as the value of number of mature berries/number of flowers × 100. The capital letter (A) above columns denotes non-significant between-year variation in the fruit set of Vaccinium myrtillus. Small letters indicate the significant difference between the control and picking treatment plots at the level p < 0.05. Results are obtained by a linear mixed model analysis. In 2013: control: N = 15, picking treatment: N = 15, and in 2014: control: N = 14, picking treatment: N = 15.
The mean fresh weight of berries was similar in the control and picking treatment plots in 2015 (one-factor ANOVA, F(1,38) = 0.990, p = 0.326). The mean fresh weight (g) of berries in the control and picking treatment plots was 0.25 ± 0.1 and 0.26 ± 0.1 (mean ± SE, N = 20), respectively, in 2015.
Total vascular plant abundance was higher in 2013 and 2014 compared to 2015 (Table 5). The picking treatment had no effect on the relative abundances of plant functional types (Table 5) (Fig. 3). However, the relative abundance of deciduous dwarf shrubs was higher in 2015 than the other years of the experiment, especially when combined with picking (Table 5) (Fig. 3). The relative abundance of graminoids was lower both in the control and picking treatment plots in 2015 than in 2013 and 2014 (Table 5) (Fig. 3). The relative abundance of mosses, litter and bare soil was similar among the years, and was not affected by picking, as revealed by the linear mixed model analysis (results not shown).
| Table 5. Results of linear mixed models testing the effect of picking treatment on the total vascular plant abundance and the relative abundance of plant functional types over years 2013‒2015. View in new window/tab. |
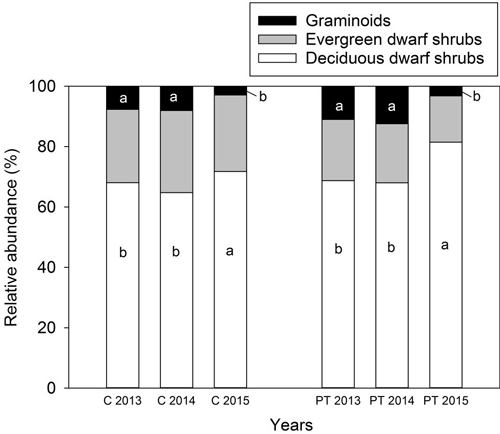
Fig. 3. Relative abundance (percentage values from the total vascular plant abundance) of plant functional types in the control and picking treatment plots in 2013–2015. Prefix “C” refers to the control and “PT” to the picking treatment on the X-axis. The picking treatment had no effect on the relative abundance of plant functional types. Small letters indicate the significant difference in the relative abundance of deciduous and graminoid species between years as revealed by a linear mixed models and LSD post hoc-test. A significant interaction between treatment and year was found for deciduous dwarf shrubs, which increased when combined with picking in 2015. A relative abundance of forbs is not shown in the figure due to their low abundance. In 2013 and 2014: control: N = 29, picking treatment: N = 30. In 2015: control: N = 20, picking treatment: N = 20.
The biomass of bilberry, pooled biomass of graminoids and the pooled biomass of other vascular plant species in the control plots were similar to the picking treatment plots in 2015, after three years of treatments (one-factor ANOVA; bilberry: F(1,10) = 1.986, p = 0.189; graminoids F(1,6) = 1.444, p = 0.275 and the pooled biomass of other vascular plant species: F(1,10) = 1.644, p = 0.229).
4 Discussion
The findings of increased fruit production and fruit set of bilberry in our study suggest enhanced bilberry yields in areas under continuous picking. These results are not in accordance with several previous studies suggesting that biomass loss and mechanical damage due to the natural herbivory (Fernández-Calvo and Obeso 2004; Hegland et al. 2005) or clipping cause reductions in bilberry fruit production and fruit set (Mathisen et al. 2010; Nestby et al. 2014; Pato et al. 2016).
A number of references support our findings of increased berry production and fruit set of bilberry after biomass loss. Tolvanen et al. (1993b) found that the potential for sexual reproduction in terms of flower production may be even higher after two to three years of delay after about a 50% bilberry biomass loss. High bilberry flower production was associated with the vigorous growth of previous years’ shoots and accelerated rejuvenation process after damage, leading to the assumption that moderate biomass loss may be even beneficial to bilberry performance (Tolvanen et al. 1993b). On the other hand, in the study by Tolvanen et al. (1993b), the enhanced flower production after the 50% biomass loss did not lead to the higher bilberry fruit production, probably due to the lack of resources for fruit maturation in already stressed plants. Besides the lack of resources, a bilberry flower has to face several challenges, such as harsh weather conditions at the several stages of berry development (Selås et al. 2015) and the availability of pollinators (Nuortila et al. 2002), before becoming a berry. In this study, the number of flowers was not significantly affected, while both fruit production and fruit set increased without an indication of reduced fruit mass after picking, and the biomass loss was fully compensated. Hence, we can only assume that perhaps some aspects of pollination, such as flower availability to pollinators, changed, while the resources for fruit maturation were still adequate after continuous picking. However, the factor behind the improved bilberry fruit production and fruit set remains unsolved in this study.
Bilberry fruit production is associated with current annual shoot size (Tolvanen and Laine 1997; Fernández-Calvo and Obeso 2004) and is linked to the ability of bilberry to produce new growth after biomass loss. In this study bilberry lost annually less than 0.5% of its total standing biomass, which is far more than 50 times lower as compared to the 25% to 100% biomass loss applied by clipping in most of the studies on bilberry performance (Tolvanen et al. 1994; Nestby et al. 2014; Pato et al. 2016). The recovery time after severe damage takes several years (Tolvanen et al. 1994; Hautala et al. 2008), during which vegetative recovery prevails and bilberry fruit production is postponed (Tolvanen et al. 1993b; Nestby et al. 2014). For example, Stark et al. (2010) reported that already a one-time 20% biomass loss from the top of the ramets of bilberry postpones bilberry fruit production for two years. This is probably because a biomass loss decreases the reproductive units of plants (Salemaa et al. 1999) or reduces the size of bilberry (Strengbom et al. 2003; Hegland et al. 2005), which induces the need for an allocation of resources to a vegetative recovery instead of sexual reproduction. However, a delay in berry production was not detected in the study by Manninen and Peltola (2013), in which about 1% of bilberry biomass was lost after intentionally powerful picking by rake.
Bilberry compensates for early growing season biomass loss more effectively than late growing season damage (Tolvanen et al. 1993). Picking-related damage is limited to 2‒3 weeks at the time when the growing season is already over and the accumulation of resources to belowground has started. A resource accumulation to belowground is crucial for producing new growth a year after damage, but it also apparently prevents the regrowth of bilberry after biomass loss in the autumn (Tolvanen et al. 1993a). This is beneficial for bilberry performance as regrowth after late damage may increase the risk of premature death of produced shoots in forthcoming years (Tolvanen et al. 1993a). Even severe aboveground damage is suggested to cause rather small resource loss in autumn, and to have no negative effect on the compensation for lost biomass in other deciduous dwarf shrub species, bog bilberry (V. uliginosum L.) (Salemaa et al. 1999). Hence it seems that the timing of picking in the late growing season may protect bilberry from strong negative effects of biomass loss on regrowth ability in next spring.
Besides the intensity and timing of biomass loss, the type of damage, i.e. the origin of the lost biomass, may affect vegetative recovery (Tolvanen et al. 1992) and, thereby, later sexual reproduction. The majority of biomass ripped from the bilberry by raking consisted of leaves and current annual shoots in our study, although the share of their biomass was not separated from the total biomass which was lost by picking. Tolvanen et al. (1992) found that losing of 50–100% of leaf biomass at the ramet level did not allow a new, photosynthesizing biomass to emerge, leading to a delay in the bilberry fruit production similarly to intensive damage by shoot cutting. Contrary to the loss of leaf biomass, removing current annual shoots breaks the apical dominance and triggers compensation for lost biomass from dormant buds in the lower parts of the bilberry ramet, being less detrimental to bilberry performance (Tolvanen et al. 1992). However, Tolvanen (1994) also found that less intense biomass loss at the beginning of the growing season does not have such a drastic effect on the regrowth of bilberry. Removing 50% of leaves from the current annual shoots (instead of from the whole ramet in Tolvanen et al. 1992) and removing 50% of all current annual shoots at the beginning of the growing season did not affect the growth and flower production of bilberry, although the author did not follow the later development of flowers into berries (Tolvanen 1994).
Picking had no effect on the relative abundance of plant functional types, revealing that damage caused by picking was too weak to affect the plant community structure. Especially graminoid wavy hair-grass responds by rapid growth to small- to large-scale damage in the understorey of boreal forests (Palviainen et al. 2005; Hautala et al. 2008). Wavy hair-grass occurs typically in low abundances within dense bilberry stands and competes with bilberry for light (Strengbom et al. 2004). Continuous damage may alleviate light availability and relax the aboveground competition between wavy hair-grass and bilberry (Strengbom et al. 2004), leading to the increased abundance of fast-growing graminoid species in the understorey (Bowman et al. 1995; Hawkes and Sullivan 2001). In our study, deciduous dwarf shrubs maintained their dominance, and also increased by picking in the understorey at the end of the experiment. Instead of investing in secondary defense metabolites, the production of new growth is suggested to be the most important strategy for bilberry to respond to biomass loss (Tolvanen and Laine 1997; Dahlgren et al. 2007; Jaakola et al. 2008). Moreover, deciduous species, such as bilberry, accumulate efficiently resources to belowground organs which enable them to relocate resources for rapid growth after damage (Tolvanen and Laine 1997).
In conclusion, we suggest that the low intensity and timing of damage act as a buffer against the adverse effects of picking on bilberry fruit production. Surprisingly both bilberry fruit production and fruit set increased after continuous picking, although the reason behind this phenomenon remains unclear in this study. Picking probably accelerated the rejuvenation process of bilberry ramets, which may be beneficial to bilberry performance in the long-term. Moreover, the relative abundance of plant functional types remained the same after three years of picking, suggesting that continuous picking does not deteriorate bilberry yields by inducing changes in a vegetation community structure. On the basis of this study, it is reasonable to anticipate that there are no indications that current intensive berry picking would not be on a sustainable level.
Acknowledgements
We thank Virve Kumpula, Esko Saastamoinen, Oskari Saastamoinen, Inga Heikkinen, Carita Kuparinen and Janette Hautaluoma for the field assistance, and two anonymous reviewers for their valuable comments and suggestions. This work was financed by European Agricultural Fund for Rural Development.
References
Boulanger-Lapointe N., Järvinen A., Rartanen R., Hermann M.T. (2017). Climate and herbivore influence on Vaccinium myrtillus over the last 40 years in northwest Lapland, Finland. Ecosphere 8(1): e01654. https://doi.org/10.1002/ecs2.1654.
Bowman W.D., Theodose T.A., Fisk M.C. (1995). Physiological and production responses of plant growth forms to increases in limiting resources in alpine tundra: implications for differential community response to environmental change. Oecologia 101(2): 217–227. https://doi.org/10.1007/BF00317287.
Dahlgren J., Oksanen L., Sjödin M., Olofsson J. (2007). Interactions between gray-sided voles (Clethrionomys rufucanus) and bilberry (Vaccinium myrtillus), their main winter food plant. Oecologia 152(3): 525–532. https://doi.org/10.1007/s00442-007-0664-8.
Eldegard K., Scholten J., Stokland J.N., Granhus A., Lie M. (2019) The influence of stand density on bilberry (Vaccinium myrtillus L.) cover depends on stand age, solar irradiation, and tree species composition. Forest Ecology and Management 432: 582‒590. https://doi.org/10.1016/j.foreco.2018.09.054.
Fernández-Calvo I.C., Obeso J.R. (2004). Growth, nutrient content, fruit production and herbivory in bilberry Vaccinium myrtillus L. along an altitudinal gradient. Forestry 77(3): 213–223. https://doi.org/10.1093/forestry/77.3.213.
Finnish Meteorological Institute. http://ilmatieteenlaitos.fi/.
Hautala H., Tolvanen A., Nuortila C. (2008). Recovery of pristine boreal forest floor community after selective removal of understorey, ground and humus layers. Plant Ecology 194(2): 273–282. https://doi.org/10.1007/s11258-007-9290-0.
Hawkes C.V., Sullivan J.J. (2001). The impact of herbivory on plants in different resource conditions: a meta-analysis. Ecology 82(7): 2045–2058. https://doi.org/10.1890/0012-9658(2001)082[2045:TIOHOP]2.0.CO;2.
Hedwall P.-O., Brunet J., Nordin A., Bergh J. (2013). Changes in the abundance of keystone forest floor species in response to changes of forest structure. Journal of Vegetation Science 24(2): 296–306. https://doi.org/10.1111/j.1654-1103.2012.01457.x.
Hegland S.J., Rydgren K., Seldal T. (2005). The response of Vaccinium myrtillus to variations in grazing intensity in a Scandinavian pine forest on the island of Svanøy. Canadian Journal of Botany 83(12): 1638–1644. https://doi.org/10.1139/b05-132.
Hegland S.J., Jongejans E., Rydgren K. (2010). Investigating the interaction between ungulate grazing and resource effects on Vaccinium myrtillus populations with integral projection models. Oecologia 163(3): 695–706. https://doi.org/10.1007/s00442-010-1616-2.
Jaakola L., Koskimäki J.J., Riihinen K.R. Tolvanen A. (2008). Effect of wounding on chalcone synthase and pathogenesis related PR-10 gene expression and content of phenolic compounds in bilberry leaves. Biologia Plantarum 52(2): 391–395. https://doi.org/10.1007/s10535-008-0082-8.
Koo A.J.K., Howe G.A. (2009). The wound hormone jasmonate. Phytochemistry 70(13–14): 1571–1580. https://doi.org/10.1016/j.phytochem.2009.07.018.
Manninen O.H., Peltola R. (2013). Effects of picking methods on the berry production of bilberry (Vaccinium myrtillus), lingonberry (V. vitis-idaea) and crowberry (Empetrum nigrum ssp. hermaphroditum) in Northern Finland. Silva Fennica 47(3) article 972. https://doi.org/10.14214/sf.972.
Mathisen K.M., Buhtz F., Danell K., Bergström R., Skarpe C., Suominen O., Persson I.-L. (2010). Moose density and habitat productivity affects reproduction, growth and species composition in field layer vegetation. Journal of Vegetation Science 21(4): 705–716. https://doi.org/10.1111/j.1654-1103.2010.01180.x.
Miina J., Hotanen J.-P., Salo K. (2009). Modelling the abundance and temporal variation in the production of bilberry (Vaccinium myrtillus L.) in Finnish mineral soil forests. Silva Fennica 43(4): 577–593. https://doi.org/10.14214/sf.181.
Nestby R., Martinussen I., Krogstad T., Uleberg E. (2014). Effect of fertilization, tiller cutting and environment on plant growth and yield of European blueberry (Vaccinium myrtillus L.) in Norwegian forest fields. Journal of Berry Research 4(2): 79‒95. https://doi.org/10.3233/JBR-140070.
Nielsen A., Totland Ø., Ohlson M. (2007). The effect of forest management operations on population performance of Vaccinium myrtillus on a landscape scale. Basic and Applied Ecology 8(3): 231–241. https://doi.org/10.1016/j.baae.2006.05.009.
Nuortila C., Tuomi J., Laine K. (2002). Inter-parent distance affects reproductive success in two clonal dwarf shrubs, Vaccinium myrtillus and Vaccinium vitis-idaea (Ericaceae). Canadian Journal of Botany 80(8): 875–884. https://doi.org/10.1139/b02-079.
Palviainen M., Finér L., Mannerkoski H., Sirpa Piirainen S., Starr M. (2005). Responses of ground vegetation species to clear-cutting in a boreal forest: aboveground biomass and nutrient contents during the first 7 years. Ecological Research 20(6): 652–660. https://doi.org/10.1007/s11284-005-0078-1.
Pato J., Obeso J.R., Ploquin E.F., Jiménez-Alfaro B. (2016). Experimental evidence from Cantabrian mountain heathlands suggests new recommendations for management of Vaccinium myrtillus L. Plant Ecology & Diversity 9(2): 199–206. https://doi.org/10.1080/17550874.2016.1176080.
Riihinen K., Jaakola L., Kärenlampi S., Hohtola A. (2008). Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ”northblue” blueberry (Vaccinium corymbosum x V. angustifolium). Food Chemistry 110(1): 156–160. https://doi.org/10.1016/j.foodchem.2008.01.057.
Salemaa M., Vanha-Majamaa I., Gardner P.I. (1999) Compensatory growth of two clonal dwarf shrubs, Arctostaphylos uva-ursi and Vaccinium uliginosum in a heavy metal polluted environment. Plant Ecology 141(1–2): 79–91. https://doi.org/10.1023/A:1009847728799.
Seldal T., Hegland S.J., Rydgren K., Rodriguez‐Saona C., Töpper J.P. (2017). How to induce defense responses in wild plant populations? Using bilberry (Vaccinium myrtillus) as example. Ecology and Evolution 7(6): 1762–1769. https://doi.org/10.1002/ece3.2687.
Selås V., Sønsteby A., Heide O.M., Opstad N. (2015). Climatic and seasonal control of annual growth rhythm and flower formation in Vaccinium myrtillus (Ericaceae), and the impact on annual variation in berry production. Plant Ecology and Evolution 148(3): 350–360. https://doi.org/10.5091/plecevo.2015.1110.
Stark S., Niva A., Martz F., Vuorela E. (2010). Sustainable gathering of juniper and bilberry shoots for natural product industry. [In Finnish with English summary]. MTT, Jokioinen. 27 p. http://urn.fi/URN:ISBN:978-952-487-269-0.
Strengbom J., Olofsson J., Witzell J., Dahlgren J. (2003). Effects of repeated damage and fertilization on palatability of Vaccinium myrtillus to grey sided voles, Clethrionomys rufocanus. Oikos 103(1): 133–141. https://doi.org/10.1034/j.1600-0706.2003.12680.x.
Strengbom J., Näsholm T., Ericson L. (2004). Light, not nitrogen limits, growth of grass Deschampsia flexuosa in boreal forest. Canadian Journal of Botany 82(4): 430–435. https://doi.org/10.1139/b04-017.
Tolvanen A. (1994). Differences in recovery between a deciduous and an evergreen ericaceous clonal dwarf shrub after simulated aboveground herbivory and belowground damage. Canadian Journal of Botany 72(6): 853–859. https://doi.org/10.1139/b94-110.
Tolvanen A., Laine K. (1997). Effects of reproduction and artificial herbivory on vegetative growth and resource levels in deciduous and evergreen dwarf shrubs. Canadian Journal of Botany 75(4): 656–666. https://doi.org/10.1139/b97-073.
Tolvanen A., Laine K., Pakonen T., Saari E., Havas P. (1992). Compensatory responses of a deciduous dwarf shrub, the bilberry (Vaccinium myrtillus L.) to simulated herbivory – some comparisons with the evergreen lingonberry (Vaccinium vitis-idaea L.). Acta Oecologia 13: 607–616.
Tolvanen A., Laine K., Pakonen T., Saari E., Havas P. (1993a). Effect of habitat and time of clipping on the recovery of the bilberry (Vaccinium myrtillus L.). Annales Botanici Fennici 30(1): 15–20. https://www.jstor.org/stable/23726337.
Tolvanen A., Laine K., Pakonen T., Saari E., Havas P. (1993b). Above-ground growth response of the bilberry (Vaccinium myrtillus L.) to simulated herbivory. Flora 188: 197–202. https://doi.org/10.1016/S0367-2530(17)32265-X.
Tolvanen A., Laine K., Pakonen T., Saari E., Havas P. (1994). Responses to harvesting intensity in a clonal dwarf shrub, the bilberry (Vaccinium myrtillus L.). Vegetatio 110(2): 163–169. https://doi.org/10.1007/BF00033396.
Total of 34 references.

