Impact of biomass harvesting on nitrogen concentration in the soil solution in hemiboreal woody ecosystems
Kļaviņš I., Bārdule A., Lībiete Z., Lazdiņa D., Lazdiņš A. (2019). Impact of biomass harvesting on nitrogen concentration in the soil solution in hemiboreal woody ecosystems. Silva Fennica vol. 53 no. 4 article id 10016. https://doi.org/10.14214/sf.10016
Highlights
- Soil solution nitrogen concentrations in whole-tree harvesting sites are higher in sites of medium to high fertility than in sites of low fertility
- In whole-tree harvesting and stem-only harvesting sites, soil solution nitrogen concentrations are highest 2 to 3 years after harvesting
- The risks of nitrogen leaching immediately after harvesting are higher in traditional forestry systems compared to short-rotation cropping.
Abstract
Considering the increasing use of wood biomass for energy and the related intensification of forest management, the impacts of different intensities of biomass harvesting on nutrient leaching risks must be better understood. Different nitrogen forms in the soil solution were monitored for 3 to 6 years after harvesting in hemiboreal forests in Latvia to evaluate the impacts of different biomass harvesting regimes on local nitrogen leaching risks, which potentially increase eutrophication in surface waters. In forestland dominated by Scots pine Pinus sylvestris L. or Norway spruce Picea abies L. (Karst.), the soil solution was sampled in: (i) stem-only harvesting (SOH), (ii) whole‐tree harvesting, with only slash removed (WTH), and (iii) whole‐tree harvesting, with both slash and stumps harvested (WTH + SB), subplots. In agricultural land, sampling was performed in an initially fertilised hybrid aspen (Populus tremula L.× P. tremuloides Michx.) short-rotation coppice (SRC), where above-ground biomass was harvested. In forestland, soil solution N (nitrogen) concentrations were highest in the second and third year after harvesting. Mean annual values in WTH subplots of medium to high fertility sites exceeded the mean values in SOH subplots and control subplots (mature stand where no harvesting was performed) for the entire study period; the opposite trend was observed for the low-fertility site. Biomass harvesting in the hybrid aspen SRC only slightly affected NO3–-N (nitrate nitrogen) and NH4+-N (ammonium nitrogen) concentrations in the soil solution within 3 years after harvesting, but a significant decrease in the TN (total nitrogen) concentration in the soil solution was found in plots with additional N fertilisation performed once initially.
Keywords
nitrogen concentration;
stump harvesting;
whole-tree harvesting;
soil solution;
hemiboreal forest;
short-rotation coppice
-
Kļaviņš,
Latvian State Forest Research Institute “Silava”, 111 Rigas Str., LV 2169, Salaspils, Latvia; University of Latvia, Raiņa blvd 19-125, LV 1586, Riga, Latvia
E-mail
ivars.klavins@silava.lv

- Bārdule, Latvian State Forest Research Institute “Silava”, 111 Rigas Str., LV 2169, Salaspils, Latvia; University of Latvia, Raiņa blvd 19-125, LV 1586, Riga, Latvia E-mail arta.bardule@silava.lv
- Lībiete, Latvian State Forest Research Institute “Silava”, 111 Rigas Str., LV 2169, Salaspils, Latvia E-mail zane.libiete@silava.lv
- Lazdiņa, Latvian State Forest Research Institute “Silava”, 111 Rigas Str., LV 2169, Salaspils, Latvia E-mail dagnija.lazdina@silava.lv
- Lazdiņš, Latvian State Forest Research Institute “Silava”, 111 Rigas Str., LV 2169, Salaspils, Latvia E-mail andis.lazdins@silava.lv
Received 21 June 2018 Accepted 17 October 2019 Published 4 November 2019
Views 103537
Available at https://doi.org/10.14214/sf.10016 | Download PDF

1 Introduction
With the ambitious targets for enlarging the proportion of renewable energy sources, set by the European Union (European Parliament 2009), the market for biomass energy is constantly growing, and this trend is expected to continue in the future. Latvia is no exception in this regard, as the National Renewable Energy Action Plan foresees to increase the share of energy produced from renewable energy sources in gross final energy consumption from 32.6% in 2005 to 40% in 2020 (Latvian Ministry of Economics 2010). To meet the growing demand, logging residues that become available after forest harvesting operations, e.g. slash, small-diameter trees and stumps, are of high interest in the countries in the Baltic Sea region.
More intensified forestry practices, however, may have adverse effects on the environment and the production of the next forest generation. A number of studies suggest that clearfelling may cause the deterioration of water quality in ground- and surface waters (Gundersen et al. 2006; Laudon 2009; Miettinen et al. 2012). Mechanical disturbance of the forest floor, associated with stump lifting, may increase the potential for nitrate and potassium leaching to ground- and surface waters as well as that of other pollutants, for example, mercury (Nieminen 2004; Munthe and Hultberg 2004; Laurén et al. 2005; Gundersen et al. 2006; Bishop et al. 2009). Potential risks associated with the long-term nutrient depletion following intensified harvesting have also been highlighted by, for example, Rolff and Ågren (1999), Merganičová et al. (2005), Thiffault et al. (2011) and others. Still, as may be concluded from the reviews by Wall (2012) and Persson (2016), the effects of whole-tree harvesting and stump lifting are largely site-and scale-dependent.
At the same time, whole-tree harvesting may be a way to reduce the load of nitrogen to forest ecosystems. Some negative effects from excessive nitrogen deposition due to air pollution during the last decades of the 20th century have been observed in Latvia. Data reported to the European Environment Agency reveal moderate pressure from critical loads of nitrogen in several parts of the country (European Environment Agency, 2017).
Excessive nitrogen (N) loads from land-based sources are one of the main causes of the eutrophication of the Baltic Sea. According to Stålnacke (1996) and Rönnberg and Bonsdorff (2004), 1 360 000 Mg of N are annually discharged into the Baltic Sea through riverine load, coastal point sources, atmospheric deposition and nitrogen fixation. A recent study (Gurinimas 2019) compiled available metadata from different economic sectors in Latvia and Estonia, and estimated that the annual leaching of total N from forest land and wetlands in Latvia to the Baltic Sea equals 14 300 Mg. On an areal basis, agricultural and urban lands export more plant nutrients than forests, but forests cover almost half of the Baltic Sea catchment area and may thus contribute to a great share of the nutrient export rates (Högbom and Futter 2013). Results of different studies show that the effects of forest management on hydrology and water quality are highly variable in both magnitude and duration. Factors such as climate, site productivity, forest type and tree-species composition, topography, sub-surface geology, watershed composition, logging system and extent of harvest may all influence the ecosystem response to whole-tree harvesting and are difficult to separate (Keenan and Kimmins 1993).
The European Union is focusing on increasing the use of renewable energy sources. One of these sources, known as short-rotation coppice (SRC), involves planting woody plants as an energy carrier on agricultural sites (Hartwich et al. 2016). The SRC with fast-growing tree species is becoming an increasingly popular option for bioenergy production due to potentially high yields, along with longer rotation periods than needed for annual plants and lower fertilisation needs when compared to other energy crops (Diaz-Pines et al. 2016). Using fast-growing trees in SRCs implies an increasing risk of depleting the soil nutrient stocks by direct biomass removal and low nutrient return (Guénon et al. 2016). Therefore, the use of fertilisers at low rates (30–75 kg N ha–1 a–1) is a common practice in SRC to increase plant biomass production through improved plant nutritional status (Bardule et al. 2013; Diaz-Pines et al. 2016).
Recommendations for inorganic fertilisation to SRC fields have been developed in different countries (Dimitriou et al. 2009; Dimitriou and Mola-Yudego 2017). However, fertilisation increases the risk of N losses due to enhanced nitrate leaching and N2O emissions (Diaz-Pines et al. 2016). Schmidt-Walter and Lamersdorf (2012) highlight that there are two stages where relatively increased amounts of nutrients might be leached from SRC cultivations: (1) when SRC are newly installed and intensive or even deep ploughing is applied before cultivation and (2) when SRCs are harvested and the export function for nutrient compounds by tree uptake and harvesting measures is offset.
Nevertheless, SRC may provide unique ecological services that warrant consideration. This approach is generally considered as the use of a crop that improves the water quality by reducing water velocity, thereby promoting infiltration, sediment deposition and nutrient retention and uptake by tree roots (Dimitriou et al. 2009; Bardule et al. 2018). The SRCs may act as physical barriers in the formation of “arable deserts” and protect against soil erosion or act as riparian or groundwater buffer strips to protect soil and water qualities in the context of the Water Framework Directive 2006/118/EU (European Parliament 2000) by reducing nutrient losses to the groundwater (Schmidt-Walter and Lamersdorf 2012). The dual ecological impact of SRC management on water issues and soil nutrient cycling is described in several publications (Dimitriou et al. 2009; Dimitriou and Mola-Yudego 2017).
The aim of this study was to evaluate the impacts of stem-only harvesting, whole-tree harvesting and stump harvesting 6 years after harvest and short-rotation crop harvesting 3 years after harvest in hemiboreal woody ecosystems in Latvia. For that, we used a combined dataset from three experiments, established to evaluate the impacts of different tree biomass harvesting regimes in forestland and agricultural land. We hypothesised that (i) in forestland, whole-tree harvesting has a greater impact on the N concentration in the soil solution than stem-only harvesting, that (ii) whole-tree harvesting in forestland has a greater impact on the N concentration in the soil solution than stump harvesting and that (iii) biomass harvesting in the SRC systems may increase N leaching risks in agricultural land.
2 Materials and methods
2.1 Description of the study sites
Temporary changes in the nitrogen concentration in the soil solution after biomass harvesting in hemiboreal conditions were evaluated in seven research sites from three different experiments, established at the same time. One site represented hybrid aspen short-rotation coppice (SRC) on agricultural land with the application of different fertilisers. Six sites represented traditional forestry with different clearfelling regimes: (i) stem-only harvesting (SOH), with slash and stumps remaining at the site; (ii) whole‐tree harvesting, with slash removed (WTH); (iii) whole‐tree harvesting, with both slash and stumps removed (WTH + SB); (iv) forested control sites without harvesting and fertilisation (C) (Table 1).
| Table 1. Description of the study sites for a study on logging effects on soil N in Latvia. SOH - stem-only harvesting, with slash and stumps remaining at the site; WTH - whole‐tree harvesting with slash removed; WTH + SB - whole‐tree harvesting, with both slash and stumps removed; C - control sites without harvesting; SRC - short-rotation coppice. | ||||||||
| Site number | Site name | Coordinates | Average annual precipitation amount, mm | Mean annual air temperature, °C | Mean temperature of coldest month, °C | Mean temperature of warmest month, °C | Site type/ Dominant tree species before harvesting | Type/year of harvesting |
| 1 | Hylocomiosa (Vilkukalns) | 56°44´N, 25°54´E | 790 | 7.0 | –7.0 (January) | +17.1 (July) | Hylocomiosa/ Pinus sylvestris L. | SOH, WTH, C/ 2013 |
| 2 | Oxalidosa turf. mel. (Kudrenis) | 56°43´N, 25°52´E | 790 | 7.0 | –7.0 (January) | +17.1 (July) | Oxalidosa turf. mel./ Picea abies L. (Karst.) | SOH, WTH, C/ 2013 |
| 3 | Myrtillosa (Zveri) | 56°40´N, 25°50´E | 790 | 7.0 | –7.0 (January) | +17.1 (July) | Myrtillosa/ Pinus sylvestris L. | SOH, WTH, C/ 2013 |
| 4 | Hylocomiosa (Rembate) | 56°47´N, 24°45´E | 789 | 7.5 | –4.9 (January) | +18.8 (July) | Hylocomiosa/ Picea abies L. (Karst.) | WTH, WTH + SB/ 2012 |
| 5 | Hylocomiosa (Dursupe) | 57°11´N, 22°56´E | 671 | 7.3 | –5.2 (January) | +17.9 (July) | Hylocomiosa/ Picea abies L. (Karst.) | WTH, WTH + SB/ 2012 |
| 6 | Hylocomiosa (Nitaure) | 57°06´N, 25°09´E | 927 | 6.7 | –5.1 (January) | +18.5 (July) | Hylocomiosa/ Picea abies L. (Karst.) | WTH, WTH + SB/ 2012 |
| 7 | Hybrid aspen SRC | 56°42´N, 25°08´E | 790 | 7.0 | –5.7 (January) | +18.5 (July) | SRC/ Populus tremula L. × P. tremuloides Michx. | WTH, C/ 2015 |
Three sites (Hylocomiosa (Vilkukalns), Oxalidosa turf. mel. (Kudrenis) and Myrtillosa (Zveri)) of the first experiment were established in experimental forests of the Kalsnava Forest district, eastern part of Latvia, to evaluate the impact of above-ground biomass removal on nutrient concentration in the soil solution (Fig. 1). Two sites were located on dry mineral soil with different fertility levels (Hylocomiosa – mesotrophic site – and Myrtillosa – oligotrophic site, as indicated by site index; the differences were obvious also when comparing ground vegetation); the third site (Oxalidosa turf. mel.) was eutrophic and located on peat soil drained in 1960. We used Latvian forest site type classification system developed by Kaspars Bušs (1981), where all site types are grouped into five groups of growing conditions (sites on dry mineral soil, sites on wet mineral soil, sites on wet peat soil, sites on drained mineral soil, sites on drained peat soil), and further arranged according to site fertility. At each site, three sampling subplots (size of the subplots varied from 0.4–0.9 ha) were established next to each other in the following order: WTH, SOH and C. Clearfelling in WTH and SOH subplots was performed in early spring 2013 with a harvester, timber was extracted and logging residues were removed with a forwarder, following the ‘business as usual’ principle. During harvest, the soil was frozen, and no significant damage to the soil due to the movement of machinery was observed. At the WTH sampling subplots, the entire above-ground part of the tree was harvested (in practice, this means that approximately 70% of treetops and branches were removed). At the SOH sampling subplots, only the stemwood was removed, and logging residues were evenly scattered throughout the plot. At the Hylocomiosa and Myrtillosa sites, the soil was prepared by disk trenching in autumn 2014, and 2-year-old pine container seedlings were planted in spring 2015. At the Oxalidosa turf. mel. site, 3-year-old spruce bareroot plants were planted in unprepared soil in spring 2015 (Tables 1–3).
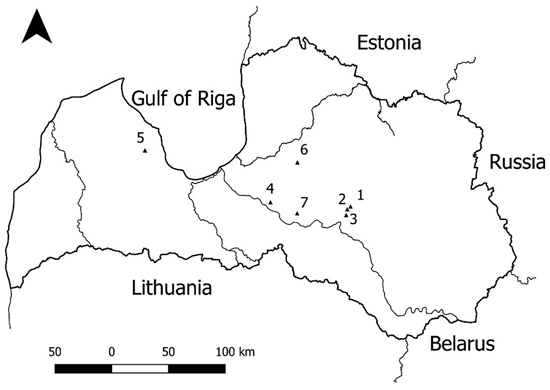
Fig. 1. Location of the study sites. 1 – Hylocomiosa (Vilkukalns); 2 – Oxalidosa turf. mel. (Kudrenis); 3 – Myrtillosa (Zveri); 4 – Hylocomiosa (Rembate); 5 – Hylocomiosa (Dursupe); 6 – Hylocomiosa (Nitaure); 7 – Hybrid aspen SRC. A study on logging effects on soil N in Latvia.
| Table 2. Experimental setup in the sites for a study on logging effects on soil N in Latvia. | ||||||
| Site number | Site name | Type of management after harvesting | Subplots | Date of installation of lysimeters | Sampling design | Frequency of soil solution sampling |
| 1 | Hylocomiosa (Vilkukalns) | Reforestation with Pinus sylvestris L. | Three subplots per site: WTH, SOH, C | autumn 2011 | three pairs of lysimeters at 2 depths (30 and 60 cm) per subplot | twice per month in 2013–2015 and once per month in 2016 and 2017 |
| 2 | Oxalidosa turf. mel. (Kudrenis) | Reforestation with Picea abies (L.) Karst. | ||||
| 3 | Myrtillosa (Zveri) | Reforestation with Pinus sylvestris L. | ||||
| 4 | Hylocomiosa (Rembate) | Reforestation with Picea abies (L.) Karst. and Alnus glutinosa (L.) Gaertn. | Two subplots per site: WTH + SB, WTH | spring 2014 | two pairs of suction tube lysimeters at 2 depths (30 and 60 cm) per subplot | twice per month in 2014 and 2015 and once per month in 2016 and 2017 |
| 5 | Hylocomiosa (Dursupe) | Reforestation with Picea abies (L.) Karst. | ||||
| 6 | Hylocomiosa (Nitaure) | Reforestation with Picea abies (L.) Karst. | ||||
| 7 | Hybrid aspen SRC | Regeneration with P. tremula L.× P. tremuloides Michx. | Four subplots per site: WS, WA, D, C | summer 2011 | one pair of suction tube lysimeters at 2 depths (30 and 60 cm) per subplot | twice per month in 2014 and 2015 and once per month in 2016 and 2017 |
| WTH - whole‐tree harvesting with slash removed; SOH - stem-only harvesting with slash and stumps remaining at the site; C - control sites without harvesting; WTH + SB - whole‐tree harvesting, with both slash and stumps removed; SRC - short-rotation coppice. C - control plot (without fertilisation); WA - initially fertilised with wood ash; WS - initially fertilised with wastewater sludge; D - initially fertilised with digestate. | ||||||
| Table 3. Soil description of the sites for a study on logging effects on soil N in Latvia. | ||||||||||
| Site number | Site name | Soil type | Soil type (WRB*) | Soil texture (FAO) | Total C concentration, g kg–1 | Total N concentration, g kg–1 | ||||
| O horizon | 0–40 cm | 40–80 cm | O horizon | 0–40 cm | 40–80 cm | |||||
| 1 | Hylocomiosa (Vilkukalns) | mineral | Folic Umbrisols (Albic, Hyperdystric, Arenic) | sand | 545.4 | 7.8 | 3.9 | 15.5 | 0.2 | 0.2 |
| 2 | Oxalidosa turf. mel. (Kudrenis) | drained peat** | Rheic Histosols (Eutric, Drainic) | sand | 555.4 | 104.6 | 46.1 | 22.1 | 5.6 | 2.4 |
| 3 | Myrtillosa (Zveri) | mineral | Albic Arenosols (Dystric) | sandy loam | 422.1 | 7.2 | 2.9 | 11.3 | 0.3 | 0.1 |
| 4 | Hylocomiosa (Rembate) | mineral | Folic Albic Podzols | sand at 0–30 cm; sandy loam at 30–45 cm; sand at 45–80 cm depth | 331.9 | 50.4 | 5.0 | 12.9 | 1.7 | 0.2 |
| 5 | Hylocomiosa (Dursupe) | mineral | Orsteinic Albic Folic Podzols | sand | 320.4 | 9.5 | 5.6 | 9.6 | 0.3 | 0.1 |
| 6 | Hylocomiosa (Nitaure) | mineral | Folic Arenosols | sand | 452.0 | 13.9 | 5.2 | 14.8 | 0.5 | 0.1 |
| 7 | Hybrid aspen SRC | mineral | Luvic Stagnic Phaeozem (Hypoalbic) and Mollic Stagnosol (Ruptic, Calcaric, Endosiltic) | loam at 0–20 cm depth; sandy loam at 20–80 cm depth | - | 17.7 | 7.2 | - | 1.3 | 0.3 |
| * - IUSS Working Group WRB (2006). World reference base for soil resources 2006. World Soil Resources Reports No. 103. FAO, Rome. ** - drainage was carried out in 1960. | ||||||||||
Three sites (Hylocomiosa (Rembate), Hylocomiosa (Dursupe) and Hylocomiosa (Nitaure)) of the second experiment were established in the Hylocomiosa forest site type in western and central parts of Latvia to evaluate the impact of stump and root biomass removal on nutrient concentration in the soil solution (Fig. 1). At each site, two sampling subplots (WTH, considered as control, and WTH + SB) were established, with a size of 0.9–1.7 ha. Between the WTH and WTH + SB sampling subplots, a buffer zone (a 10-m-wide zone where stump and root biomass was removed and a 10-m-wide zone where stump and root biomass was left) was established. Stump and root biomass harvesting was performed in winter 2012, using two types of stump extraction scoops: the CBI stump extraction scoop mounted on a Komatsu PC210LC excavator and the stump extraction scoop MCR-500 prototype constructed in Latvia and mounted on a New Holland E215B excavator. Small stumps were extracted and then split; during the extraction of larger stumps, one or two side roots were cut, the stump was extracted and split into two parts. Previously cut roots were then also extracted from the soil. Residual soil was removed either by shaking the stumps or dropping them down from a height of 4–5 m. In 2013 (3 to 6 months after harvesting), the harvested stump and root biomass was forwarded to the roadside for storage. Subsequently, soil preparation using active disc plough was performed, and spruce container seedlings as well as black alder and spruce bareroot saplings with improved root system were planted. The same soil scarification methods and soil preparation intensities were applied in both the control (WTH) and extracted plots (WTH + SB) (Tables 1–3).
The hybrid aspen SRC site of the third experiment was located in the central part of Latvia (Fig. 1). An experimental plot was established on agricultural land in spring 2011 as a part of a large-scale multifunctional plantation of short rotation energy crops and deciduous trees, with a total area of 16 ha. In 2011, 1-year-old hybrid aspen (P. tremula L.× P. tremuloides Michx.) seedlings (clone No. 4), grown in the Kalsnava nursery of JSC Latvia’s State Forest, Latvia, were planted with an average distance between the trees of 2.0 × 2.0 m. Two replicates of four different fertilisation subplots (the size of each subplot was 0.072 ha) were established: control with no fertilisation (C), wastewater sludge (WS), wood ash (WA) and digestate (D). Class I (according to the regulations of the Cabinet of Ministers of the Republic of Latvia No. 362) wastewater sludge (dose 10 Mg ha–1 dry matter) from “Aizkraukles ūdens” (Aizkraukle Water) and stabilised wood ash from the boiler house in Sigulda (dose 6 Mg ha–1 dry matter) were spread mechanically prior to planting. Digestate (as a point-source fertiliser at a dose of 30 Mg ha–1 fresh mass) from the methane reactor in Vecauce district (Latvia) was applied immediately after planting of the hybrid aspen seedlings. The heavy metal target values and precautionary limits were not exceeded in fertilised soils according to legislative regulations for soil and ground quality (Regulations of the Cabinet of Ministers of the Republic of Latvia No. 804) (Bardule et al. 2013, 2016). All above-ground biomass was harvested in November 2015 with a Nordic Biomass Stemster (Tables 1–4). After biomass harvesting, the site was allowed to coppice. Fertilization was not repeated.
| Table 4. Macronutrient input through fertilisation in the hybrid aspen short-rotation coppice site in a study on logging effects on soil N in Latvia. | |||
| Fertilizer | Ntotal, kg ha–1 | Ptotal, kg ha–1 | Ktotal, kg ha–1 |
| Wood ash | 2.6 | 65 | 190 |
| Sewage sludge | 259 | 163 | 22 |
| Digestate | 69 | 1.2 | 99 |
The climate of all research sites was considered continental, compared to other regions of Latvia, although the meteorological conditions differed to some extent; the site Hylocomiosa (Nitaure) had the highest annual precipitation within the study period, and the sites Hylocomiosa (Vilkukalns), Oxalidosa turf. mel. (Kudrenis) and Myrtillosa (Zveri) experienced the lowest annual temperatures both in the warmest and coldest month of the year (Table 1).
2.2 Soil solution sampling and analyses
In each of the subplots representing all treatments and the control, there were one to five pairs of suction lysimeters, depending on site, with soil solution sampler cups made of porous ceramic (92% pure Al2O3) and a body of trace metal-free PVC installed vertically into the soil (Table 2). Soilmoisture 1900L12-B02M2 and 1900L24-B02M2 soil moisture samplers were used in Hylocomiosa (Vilkukalns), Oxalidosa turf. mel. (Kudrenis) and Myrtillosa (Zveri), Hylocomiosa (Rembate), Hylocomiosa (Dursupe) and Hylocomiosa (Nitaure) sites, and Eijkelkamp 12.03 soil moisture samplers with a large ceramic cup were used in the hybrid aspen SRC plantation. In all sites, the soil solution was sampled during the growing season (April–October). The suction usually worked during all days of the month (with a few exceptions), and the lysimeters did not overload, although there were cases when during the driest months, some of the lysimeters were empty.
The soil solution samples were analysed in the Forest Environment Laboratory at the Latvian State Forest Research Institute “Silava”. Total nitrogen (TN) and nitrate nitrogen (NO3–-N) concentrations in water samples were determined using a FORMACSHT TOC/TN Analyzer (ND25 nitrogen detector, made in the Netherlands), and ammonium nitrogen (NH4+-N) was determined using the spectrometric method according to ISO 7150-1:1984. Prior to the chemical analyses, the water samples were filtered using borosilicate glass fibre filters without a binder.
2.3 Statistical analysis
Data processing and all statistical analyses were performed in the R environment (R Core Team 2017). For all forest sites, the mean annual concentrations of different N forms in the soil solution in the treated subplots (SOH and WTH subplot in Hylocomiosa (Vilkukalns), Oxalidosa turf. mel. (Kudrenis) and Myrtillosa (Zveri) sites, WTH + SB subplots in Hylocomiosa (Rembate), Hylocomiosa (Dursupe) and Hylocomiosa (Nitaure) were compared to those of the control subplots of the sites (C plot in Vilkukalns, Kudrenis and Zveri, WTH subplots in sites Rembate, Dursupe and Nitaure). To calculate the mean annual concentrations of different N forms in the soil solution in treated and control subplots, first, a sampling month average was calculated, which was then used to calculate the annual average. In addition, for sites Vilkukalns, Kudrenis and Zveri, the mean annual concentrations of different N forms in the soil solution in the subplots of different harvest intensities (SOH and WTH subplots) were compared. For the SRC site, the mean annual concentrations of different N forms in the soil solution from harvested subplots were compared to those of the control (unharvested) subplots. For this site, the data were divided into four groups according to the applied fertiliser. There were no statistically significant differences in NO3–-N, NH4+-N and TN concentrations in the soil solution between the depths of 30 and 60 cm within each study site and year; consequently, we combined data from both soil solution sampling depths in a single statistical analysis.
We used the results of repeated measures analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) test to assess the significance of treatment means of monitored chemical parameters in the soil solution. In addition, statistical differences in different N forms in the soil solution between treated and control plots within each site and each year were analysed with the Wilcoxon rank sum test with continuity corrections; the choice of the non-parametrical statistical method was justified by the non-normal distribution of the data. We used a 95% confidence level in all analyses. The results pertaining to the impact of harvesting during the first 2 years after felling (2013 and 2014) in the sites located in the Kalsnava forest district (Hylocomiosa (Vilkukalns), Oxalidosa turf. mel. (Kudrenis) and Myrtillosa (Zveri)) have been analysed using the same statistical methods and have been published by Libiete et al. (2017).
3 Results
3.1 Impact of above-ground biomass harvest in forestland
In the first 6 years after above-ground biomass harvesting, the annual average NO3–-N concentration in the soil solution in the SOH subplots ranged from 0.66 mg l–1 (Hylocomiosa (Vilkukalns), in the fifth year after harvesting) to 6.18 mg l–1 (the same site, in the third year after harvesting), while in the WTH subplots, the annual average NO3–-N concentration in the soil solution ranged from 0.02 mg l–1 (Myrtillosa (Zveri), in the sixth year after harvesting) to 10.72 mg l–1 (Oxalidosa turf. mel. (Kudrenis), in the second year after harvesting) (Table 5). The annual average proportion of N in NO3– of the TN content in the soil solution ranged from 1.1% (Hylocomiosa (Vilkukalns), unharvested plot, in the third year after harvesting) to 94.6% (the same site, SOH subplot, in the third year after harvesting). In several harvested subplots (SOH and WTH subplots at Hylocomiosa (Vilkukalns), WTH subplot at Oxalidosa turf. mel. (Kudrenis) and SOH subplot at Myrtillosa (Zveri)) the highest proportion of N in NO3– form was found in the third year after harvesting. In both Myrtillosa and Hylocomiosa, the concentration of NO3–-N in the unharvested control subplot was considerably less variable than in both harvested subplots (Fig. 2). In both sites the mean annual soil solution NO3–-N concentration was significantly higher in the SOH subplot than in the unharvested control during the entire study period (p < 0.001; Table 6).
| Table 5. Mean annual NO3–-N, NH4+-N and total N concentrations in the soil solution in the sites where above-ground biomass was harvested in a study on logging effects on soil N in Latvia. View in new window/tab. |
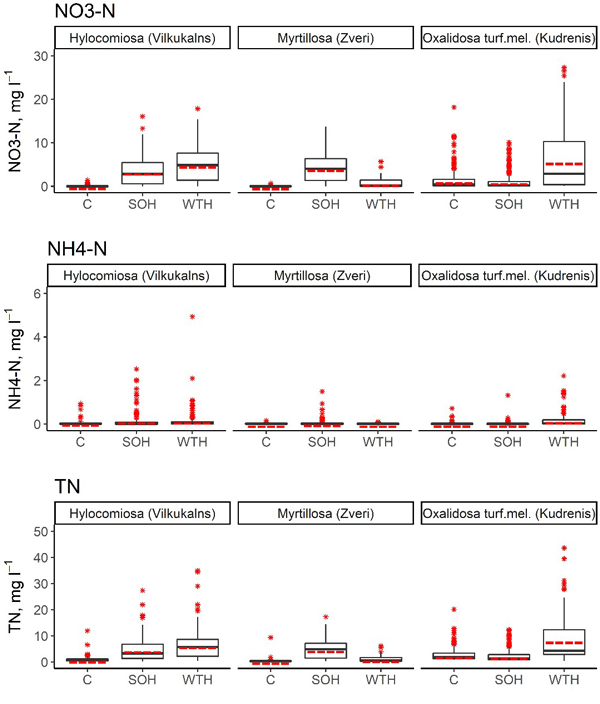
Fig. 2. Concentrations of NO3–-N, NH4+-N and total N in the soil solution in SOH (stem-only harvesting with slash and stumps remaining at the site), WTH (whole‐tree harvesting with both slash and stumps removed) and unharvested control subplots. In the boxplots, the median is shown by the black bold line, the mean is shown by the red dashed line, the box corresponds to the lower and upper quartiles, whiskers show the minimum and maximum values (within 150% of the interquartile range from the median) and red stars represent outliers of the datasets. A study on logging effects on soil N in Latvia.
| Table 6. P-values of the Wilcoxon rank sum test characterising the significance of statistical differences in mean annual NO3–-N, NH4+-N and total N concentration in the soil solution between SOH (stem-only harvesting with slash and stumps remaining at the site) or WTH (whole‐tree harvesting with slash removed) plots and C plots (control sites without harvesting). A study on logging effects on soil N in Latvia. | |||||||
| Site name | Subplot | 1st year (2013) | 2nd year (2014) | 3rd year (2015) | 4th year (2016) | 5th year (2017) | 6th year (2018) |
| NO3–-N concentration in soil solution | |||||||
| Hylocomiosa (Vilkukalns) | SOH | 0.033 | <0.001 | <0.001 | <0.001 | <0.001 | 0.009 |
| WTH | 0.110 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | |
| Oxalidosa turf. mel. (Kudrenis) | SOH | 0.200 | 0.531 | 0.748 | 0.017 | <0.001 | 0.428 |
| WTH | <0.001 | <0.001 | <0.001 | 0.173 | 0.087 | 0.090 | |
| Myrtillosa (Zveri) | SOH | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| WTH | <0.001 | <0.001 | 0.001 | 0.002 | 0.999 | 0.799 | |
| NH4+-N concentration in soil solution | |||||||
| Hylocomiosa (Vilkukalns) | SOH | 0.220 | 0.034 | 0.061 | 0.888 | 0.587 | 0.999 |
| WTH | 0.285 | <0.001 | 0.518 | 0.583 | 0.229 | 0.877 | |
| Oxalidosa turf. mel. (Kudrenis) | SOH | 0.315 | 0.575 | 0.004 | 0.999 | 0.453 | 0.047 |
| WTH | 0.002 | <0.001 | 0.033 | 0.001 | 0.001 | 0.758 | |
| Myrtillosa (Zveri) | SOH | 0.566 | 0.007 | 0.045 | 0.306 | 0.121 | 0.286 |
| WTH | 0.357 | 0.405 | 0.468 | 0.054 | 0.267 | 0.277 | |
| TN concentration in soil solution | |||||||
| Hylocomiosa (Vilkukalns) | SOH | 0.013 | <0.001 | <0.001 | <0.001 | 0.275 | 0.071 |
| WTH | 0.061 | <0.001 | <0.001 | <0.001 | 0.003 | 0.053 | |
| Oxalidosa turf. mel. (Kudrenis) | SOH | 0.765 | 0.062 | 0.347 | 0.016 | 0.001 | 0.029 |
| WTH | <0.001 | <0.001 | <0.001 | 0.999 | 0.378 | 0.106 | |
| Myrtillosa (Zveri) | SOH | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.197 |
| WTH | <0.001 | 0.001 | 0.047 | 0.198 | 0.540 | 0.291 | |
The annual average NH4+-N concentration in the soil solution in the SOH subplots reached up to 0.39 mg l–1 (Hylocomiosa (Vilkukalns), in the second year after harvesting), while in the WTH subplots, the annual average NH4+-N concentration in the soil solution amounted to 0.43 mg l–1 (the same site, in the second year after harvesting) (Table 5). The annual average proportion of N in NH4+ form of the TN content in the soil solution ranged from 0.2% (Myrtillosa (Zveri), SOH subplot, in the fourth year after harvesting) to 16.6% (Hylocomiosa (Vilkukalns), unharvested plot, in 2014). An impact of biomass harvesting on the proportion of N in NH4+ form in the soil solution was not detected. In all sites, the NH4+-N concentration varied considerably less than the NO3–-N concentration, both in the unharvested control subplot and the harvested subplots (Fig. 2). The most pronounced mean annual NH4+-N concentration differences were detected in the Oxalidosa turf. mel. (Kudrenis) site between the WTH and the control subplot (Table 6).
The annual average TN concentration in the soil solution in the SOH subplots ranged from 1.24 mg l–1 (Hylocomiosa (Vilkukalns), in the fifth year after harvesting) to 9.37 mg l–1 (the same site, in the second year after harvesting), but in the WTH subplots, the annual average TN concentration in the soil solution ranged from 0.41 mg l–1 (Myrtillosa (Zveri), in the fourth year after harvesting) to 15.16 mg l–1 (Oxalidosa turf. mel (Kudrenis), in the second year after harvesting) (Table 5). In the Hylocomiosa (Vilkukalns) and Myrtillosa (Zveri) sites (dry mineral soil), the mean annual TN concentrations were lowest in the unharvested control subplots, while in the Oxalidosa turf. mel. (Kudrenis) site, the TN concentration in the SOH subplot was slightly lower than that in the unharvested subplot. In more fertile sites (Hylocomiosa (Vilkukalns) and Oxalidosa turf. mel. (Kudrenis)), the TN concentration was highest in the WTH subplot, while in the oligotrophic site (Myrtillosa (Zveri)), the TN concentration in the soil solution was highest in the SOH subplot (Fig. 2).
Generally, the differences in NO3–-N concentrations between harvested and control plots started to increase immediately after harvesting, and the nitrate concentrations remained elevated almost in all harvested plots. The highest NO3–-N concentrations in the harvested plots were observed in the second and third year after harvesting (Table 5). In the third and fourth year after felling, NO3–-N concentrations in the soil solution started to decrease, and after 6 years of observations, they fell below the values of the unharvested control plot in the Oxalidosa turf. mel. (Kudrenis) site, but remained elevated in both sites on dry mineral soils (Table 5). In the Hylocomiosa (Vilkukalns) site, the trends describing the differences from the unharvested control were rather similar in both harvesting regimes. In the Oxalidosa turf. mel. (Kudrenis) and Myrtillosa (Zveri) sites, more explicit differences between SOH and WTH subplots were observed, but the soil solution NO3–-N concentrations in the Oxalidosa turf. mel. (Kudrenis) site were higher in the WTH subplot samples, while in the Myrtillosa (Zveri) site, they were higher in the SOH subplot samples. The differences from the unharvested control subplot were in most cases statistically significant (Table 6).
A considerable increase in the NH4+-N concentration after harvesting was observed only in the WTH subplot of the Oxalidosa turf. mel. (Kudrenis) site (with highest mean annual concentrations in the second year following harvesting, but with concentration values exceeding those in the control plot during the entire observation period) (Table 5, Fig. 2). In the case of NH4+-N, the differences from the control were in most cases not significant (Table 6).
The differences in the TN concentration from the unharvested control, in most cases, increased immediately after harvesting, and the concentrations remained elevated for most of the observation period, with highest mean annual values in the second year after harvesting. Differences from this trend were detected in the SOH subplot of the Oxalidosa turf. mel. (Kudrenis) site, where the TN concentration after harvesting increased only slightly and then decreased below the control values, and in the WTH subplot of the Myrtillosa (Zveri) site, where the TN concentration increased immediately after harvesting and decreased in the second year after felling, remaining only slightly elevated above the control level for the remaining observation period (Table 5). Except for these two cases, the differences in the TN concentrations between the treated subplots and the control subplots were mostly significant (Table 6). In contrast, no statistically significant differences in NO3–-N, NH4+-N and TN concentration between WTH and SOH subplots were detected in 2013 and 2014 in the Hylocomiosa (Vilkukalns) site (as previously published by Libiete et al. (2017)); from 2015 to 2017, the mean annual NO3–-N and TN concentrations in the soil solution of the WTH subplot were significantly higher than those in the SOH subplot (Table 5).
3.2 Impact of stump harvesting in forestland
In the first 5 years after stump harvesting, the annual average NO3–-N concentration in the soil solution in the WTH-SB subplots ranged from 0.002 mg l–1 (Rembate, in the fourth year after harvesting) to 1.43 mg l–1 (Nitaure, in the fourth year after harvesting) (Table 7). The mean NO3–-N concentrations in the Dursupe and Rembate sites were rather similar in both studied treatments in the observation period, and their variation was minor. In the Nitaure site, the mean NO3–-N concentration in the WTH-SB subplot in the fourth and fifth year of the observation period was higher than that in the WTH subplot, and the variation of values was considerably more pronounced (Table 7, Fig. 3).
| Table 7. Mean annual NO3–-N, NH4+-N and total N concentrations in the soil solution in study sites where stumps were harvested (WTH + SB - all above-ground biomass (including slash) and stumps removed; WTH - only above-ground biomass (including slash) removed). 2014 represents the first year after harvesting. | ||||||
| Year | Hylocomiosa (Rembate) | Hylocomiosa (Dursupe) | Hylocomiosa (Nitaure) | |||
| WTH + SB | WTH | WTH + SB | WTH | WTH + SB | WTH | |
| NO3–-N concentration in soil solution ± standard error, mg l–1 | ||||||
| 2014 | 0.10 ± 0.05 | 0.09 ± 0.05 | 0.10 ± 0.07 | 0.05 ± 0.03 | 0.86 ± 0.29 | 1.69 ± 0.86 |
| 2015 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.05 ± 0.02 | 0.49 ± 0.19 | 0.45 ± 0.24 |
| 2016 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.02 | 0.05 ± 0.02 | 0.47 ± 0.18 | 0.42 ± 0.23 |
| 2017 | <0.01* | 0.05 ± 0.03 | 0.03 ± 0.02 | 0.06 ± 0.02 | 1.43* ± 0.54 | 0.09 ± 0.04 |
| 2018 | 0.01 ± 0.01 | <0.01 | <0.01 | <0.01 | 1.04 ± 0.67 | 0.10 ± 0.03 |
| NH4+-N concentration in soil solution ± standard error, mg l–1 | ||||||
| 2014 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02* ± 0.01 | 0.81 ± 0.23 | 0.03 ± 0.01 | 0.15 ± 0.11 |
| 2015 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.23 ± 0.14 | 0.34 ± 0.08 | 0.01 ± 0.01 | 0.04 ± 0.02 |
| 2016 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.22 ± 0.13 | 0.33 ± 0.09 | 0.01 ± 0.01 | 0.04 ± 0.02 |
| 2017 | 0.21 ± 0.07 | 0.48 ± 0.07 | 0.17 ± 0.05 | 0.26 ± 0.13 | 0.32 ± 0.12 | 0.02 ± 0.01 |
| 2018 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.03 | 0.01 ± 0.01 |
| TN concentration in soil solution ± standard error, mg l–1 | ||||||
| 2014 | 0.53 ± 0.05 | 0.50 ± 0.08 | 2.47 ± 0.75 | 2.18 ± 0.29 | 1.19 ± 0.23 | 2.77 ± 1.08 |
| 2015 | 0.36 ± 0.03 | 0.30 ± 0.03 | 1.82 ± 0.26 | 1.32 ± 0.20 | 0.80* ± 0.20 | 1.13 ± 0.23 |
| 2016 | 0.34 ± 0.18 | 0.26 ± 0.02 | 1.69 ± 0.24 | 1.23 ± 0.19 | 0.77 ± 0.19 | 1.01 ± 0.19 |
| 2017 | 0.76 ± 0.18 | 2.08 ± 0.65 | 1.52 ± 0.34 | 1.12 ± 0.17 | 2.76* ± 0.80 | 0.36 ± 0.04 |
| 2018 | 0.44 ± 0.04 | 0.52 ± 0.09 | 0.98 ± 0.28 | 0.59 ± 0.04 | 1.73 ± 0.70 | 0.43 ± 0.05 |
| * Statistically significant difference between WTH + SB and WTH subplots within year by the Wilcoxon rank sum test. | ||||||
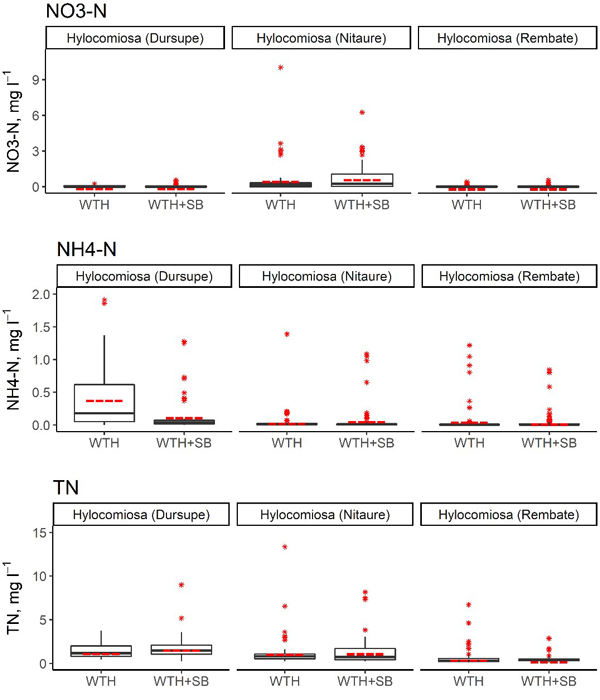
Fig. 3. Concentrations of NO3–-N, NH4+-N and total N in the soil solution in WTH + SB (above-ground biomass (including slash) and stumps harvested) and WTH (above-ground biomass (including slash) harvested). In the boxplots, the median is shown by the black bold line, the mean is shown by the red dashed line, the box corresponds to the lower and upper quartiles, whiskers show the minimal and maximal values (within 150% of the interquartile range from the median) and red stars represent outliers of the datasets. A study on logging effects on soil N in Latvia.
No significant differences were observed between WTH and WTH + SB subplots in the context of annual mean NO3–-N concentrations in the soil solution, except for Rembate and Nitaure sites in 2017 (Table 8). In the Nitaure site, the NO3–-N concentrations after the harvest differed considerably from those of the WTH subplot in 2014 and 2017, while minor differences between WTH + SB and WTH treatments were observed for the Rembate and Dursupe sites (Table 7). In the Nitaure site, a 16 times higher NO3–-N concentration (1.43 ± 0.54 mg l–1, 13 samples) in the WTH + SB subplot than in the WTH subplot (0.088 ± 0.040 mg l–1, 8 samples) was observed in 2017. On the contrary, in the Rembate site, a 30 times lower concentration (0.002 mg l–1, 16 samples) was observed in the WTH + SB subplot in the same year. Extremely low mean annual values of NO3–-N were detected also in the Rembate site, WTH subplot, and in both treatments of the Dursupe site in 2018. In general, the NO3–-N concentration in the soil solution fluctuated between 0.001 and 1.841 mg l–1, causing hyped proportional differences because of low values. The annual average proportion of N in NO3– form in the soil solution ranged from 0.4% (Rembate, WTH + SB subplot, in the fourth year after harvesting) to 52.9% (Nitaure, WTH + SB subplot, in the first year after harvesting) of the TN content. Furthermore, in the Nitaure site, throughout the entire observation period, a higher proportion of N in NO3– form was found in the WTH + SB subplot in comparison to the WTH subplot, although statistically significant differences in the proportion of N in NO3– form between WTH + SB and WTH subplots were not detected.
| Table 8. P-values of the Wilcoxon rank sum test characterising the significance of statistical differences in mean annual NO3–-N, NH4+-N and total N concentrations in the soil solution between WTH + SB (whole‐tree harvesting with both slash and stumps removed) and WTH (whole‐tree harvesting with slash removed) plots. | |||||
| Year after harvesting | 1st year (2014) | 2nd year (2015) | 3rd year (2016) | 4th year (2017) | 5th year (2018) |
| NO3–-N concentration in soil solution | |||||
| Hylocomiosa (Rembate) | 0.681 | 0.604 | 0.378 | 0.013 | 0.187 |
| Hylocomiosa (Dursupe) | 0.825 | 0.136 | 0.340 | 0.443 | 0.505 |
| Hylocomiosa (Nitaure) | 0.782 | 0.956 | 0.956 | 0.015 | 0.603 |
| NH4+-N concentration in soil solution | |||||
| Hylocomiosa (Rembate) | 0.828 | 0.874 | 0.781 | 0.379 | 0.812 |
| Hylocomiosa (Dursupe) | <0.001 | 0.276 | 0.276 | 0.999 | 0.857 |
| Hylocomiosa (Nitaure) | 0.253 | 0.201 | 0.253 | 0.143 | 0.999 |
| TN concentration in soil solution | |||||
| Hylocomiosa (Rembate) | 0.496 | 0.127 | 0.154 | 0.560 | 0.489 |
| Hylocomiosa (Dursupe) | 0.623 | 0.051 | 0.051 | 0.770 | 0.700 |
| Hylocomiosa (Nitaure) | 0.165 | 0.0362 | 0.053 | 0.001 | 0.167 |
The annual average NH4+-N concentration in the soil solution in the WTH-SB subplots reached up to 0.34 mg l–1 (Dursupe, in the second year after harvesting), but in the WTH treatment, the highest annual average NH4+-N concentration in the soil solution was detected at the Dursupe site in the first year after harvesting (0.81 ± 0.23 mg l–1). The highest mean annual concentrations of NH4+-N within the entire observation period were observed in the WTH subplot of the Rembate site (Table 7, Fig. 3). In the other two sites, the NH4+-N concentrations in both treatments were rather similar (Fig. 3). The only statistically significant difference between the annual mean NH4+-N concentrations in the treatments was observed in the Dursupe site in the first year after harvesting (p < 0.001; Table 8). The annual average proportion of N in NH4+ form of the TN concentration in the soil solution ranged from 1.1% (Dursupe, WTH + SB subplot, in the first year after harvesting) to 30.2% (Dursupe, WTH subplot, in the first year after harvesting).
The annual average TN concentration in the soil solution in the WTH-SB subplots ranged from 0.34 mg l–1 (Rembate, in the third year after harvesting) to 2.76 mg l–1 (Nitaure, in the fourth year after harvesting), while in the WTH subplots, it ranged from 0.26 mg l–1 (Rembate, in the third year after harvesting) to 2.77 mg l–1 (Nitaure, first year after harvesting) (Table 7). The mean values of TN concentration in all sites throughout the observation period differed only slightly between the treatments, and the variation of the values was most pronounced in the Dursupe site (Fig. 3). Mostly no significant differences were observed between WTH and WTH + SB subplots in the context of annual mean TN concentrations in the soil solution, except for the Nitaure site in 2015 and 2017. However, these differences were not consistent: in the second year after harvesting, the mean annual TN concentration in the WTH + SB subplot was lower than the mean annual concentration in the WTH subplot, but in the fourth year after harvesting, the mean annual TN concentration in the WTH + SB subplot exceeded that of the WTH subplot in the same year (p-values 0.0362 and 0.001, respectively) (Tables 7 and 8).
3.3 Impact of above-ground biomass harvest in SRC
Our study covered 3 years after the first biomass harvesting of 5-year-old hybrid aspen SRC. In this period the site was allowed to coppice and fertilization was not repeated. In the first 3 years after biomass harvesting, the annual average NO3–-N concentration in the soil solution in the harvested subplots reached 0.50 mg l–1 (subplot initially fertilised with wood ash). The annual average NH4+-N concentration in the soil solution in the harvested subplots reached 0.03 mg l–1 (subplot initially fertilised with digestate), but the annual average TN concentration in the soil solution in the harvested subplots ranged from 0.42 mg l–1 (plot initially fertilised with digestate) to 1.14 mg l–1 (plot initially fertilised with wood ash) (Table 9, Fig. 4). The annual average proportion of N in NO3– of the TN content in the soil solution reached 26.5% (control subplot without fertilisation, in the third year after harvesting). Furthermore, in the first year after biomass harvesting, a higher proportion of N in NO3– form was found in all unharvested subplots in comparison to the harvested subplots, but this trend did not persist in the second and third year after harvesting.
| Table 9. Mean annual NO3–-N, NH4+-N and total N concentrations in the soil solution in the SRC site where different fertilisers were initially used. 2016 represents the first year after harvesting. | ||||||||
| Year | Digestate | Sewage sludge | Wood ash | Control (without fertilization) | ||||
| harvested | control | harvested | control | harvested | control | harvested | control | |
| NO3–-N concentration in soil solution ± standard error, mg l–1 | ||||||||
| 2016 | 0.03 ± 0.01 | 0.06 ± 0.03 | 0.02 ± 0.01 | 0.19 ± 0.09 | 0.03 ± 0.01 | 0.05 ± 0.03 | 0.06 ± 0.03 | 0.05 ± 0.03 |
| 2017 | 0.01 ± 0.01 | 0.17 ± 0.10 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.05 ± 0.03 | 0.14 ± 0.06 | 0.02 ± 0.01 |
| 2018 | <0.01 | 0.18 ± 0.04 | 0.07 ± 0.06 | <0.01 | 0.50 ± 0.80 | 0.08 ± 0.03 | 0.19 ± 0.19 | 0.10 ± 0.03 |
| NH4+-N concentration in soil solution ± standard error, mg l–1 | ||||||||
| 2016 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.03 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 |
| 2017 | 0.02 ± 0.01 | 0.02 ± 0.01 | <0.01 | <0.01 | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| 2018 | <0.01 | 0.01 ± 0.01 | 0.03 ± 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 ± 0.01 |
| TN concentration in soil solution ± standard error, mg l–1 | ||||||||
| 2016 | 0.55* ± 0.06 | 1.12 ± 0.25 | 0.68* ± 0.05 | 1.23 ± 0.06 | 0.76* ± 0.04 | 0.59 ± 0.05 | 0.90 ± 0.08 | 0.77 ± 0.08 |
| 2017 | 0.42* ± 0.03 | 1.20 ± 0.12 | 0.69 ± 0.05 | 0.75 ± 0.07 | 0.66 ± 0.06 | 0.43 ± 0.05 | 0.84* ± 0.06 | 0.57 ± 0.06 |
| 2018 | 0.42 ± 0.11 | 0.87 ± 0.02 | 0.91 ± 0.24 | 0.99 ± 0.12 | 1.14 ± 0.42 | 0.30 ± 0.12 | 1.09 ± 0.27 | 0.85 ± 0.32 |
| * Statistically significant difference between harvested and control subplots within year by the Wilcoxon rank sum test. | ||||||||
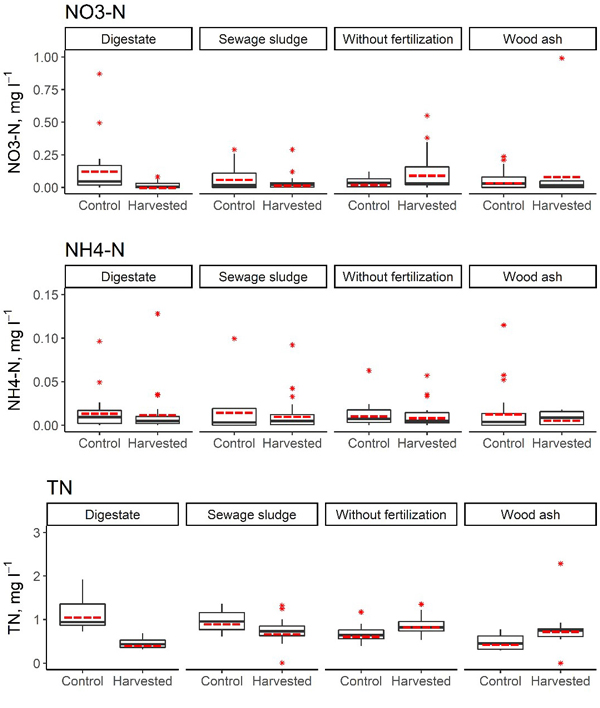
Fig. 4. Concentrations of NO3–-N, NH4+-N and total N in the soil solution in harvested and control subplots in the short-rotation coppice site with initial fertilisation. In the boxplots, the median is shown by the black bold line, the mean is shown by the red dashed line, the box corresponds to the lower and upper quartiles, whiskers show the minimal and maximal values (within 150% of the interquartile range from the median) and red stars represent outliers of the datasets. A study on logging effects on soil N in Latvia.
There were no statistically significant differences in terms of the average NO3–-N and NH4+-N concentrations in the soil solution between harvested and unharvested subplots in the first three years after harvesting (p > 0.067), but significant differences in the average TN concentration in the soil solution between harvested and unharvested subplots were found in all initially fertilised plots in 2016 (with p values ranging from 0.014 to 0.032, Table 10). The trends in differences in the TN concentration in soil solutions between harvested and unharvested subplots differed depending on the initially used fertiliser. In the first year after biomass harvesting, in subplots initially fertilised with wood ash, a higher TN concentration in the soil solution was found in the harvested subplots; in contrast, in subplots initially fertilised with digestate or sewage sludge, a significantly higher TN concentration in the soil solution was found in unharvested subplots. Also in the second year after biomass harvesting, a significantly higher TN concentration in the soil solution was found in unharvested subplots initially fertilised with digestate (p < 0.001), but in the control subplots (unfertilised), a significantly higher TN concentration in the soil solution was found in the harvested subplots (p = 0.011). The most pronounced difference in average TN concentration in the soil solution was found between harvested and unharvested subplots initially fertilised with digestate or sewage sludge, where also comparatively largest amounts of total N had been applied.
| Table 10. P-values of the Wilcoxon rank sum test characterising the significance of statistical differences between mean annual NO3–-N, NH4+-N and total N concentrations in the soil solution in harvested and unharvested plots. | ||||
| Year after harvesting | Type of fertilizer | |||
| Digestate | Sewage sludge | Wood ash | Control (without fertilization) | |
| NO3–-N concentration in soil solution | ||||
| 1st year (2016) | 0.668 | 0.246 | 0.999 | 0.999 |
| 2nd year (2017) | 0.175 | 0.520 | 0.564 | 0.181 |
| 2018 | 0.999 | 0.729 | 0.999 | 0.999 |
| NH4+-N concentration in soil solution | ||||
| 1st year (2016) | 0.133 | 0.304 | 0.067 | 0.667 |
| 2nd year (2017) | 0.563 | 0.999 | 0.604 | 0.999 |
| 2018 | 0.999 | 0.696 | 0.999 | 0.999 |
| TN concentration in soil solution | ||||
| 1st year (2016) | 0.014 | 0.022 | 0.032 | 0.517 |
| 2nd year (2017) | <0.001 | 0.839 | 0.054 | 0.011 |
| 2018 | 0.999 | 0.999 | 0.999 | 0.667 |
As the hybrid aspen SRC site showed similar fertility conditions than the Hylocomiosa sites and the Myrtillosa (Zveri) site, the soil solution N concentration between those sites was also compared (first 3-year data after harvesting). Despite the initial application of N-containing fertilisers (up to 259 kg N ha–1) in the hybrid aspen SRC site, the mean NO3–-N and TN concentrations in the soil solution during three subsequent years after biomass harvesting were significantly lower (average NO3–-N concentration was 0.06 mg l–1, average TN concentration was 0.72 mg l–1) than in several subplots in harvested forestland corresponding to the Hylocomiosa forest type (Vilkukalns, Zveri, Dursupe, Nitaure), where the mean NO3–-N concentrations in the soil solution during three subsequent years after biomass harvesting reached 8.74 mg NO3–-N l–1, but the mean TN concentrations in the soil solution reached 11.09 mg l–1 (both in Hylocomiosa (Vilkukalns), WTH subplot). It may be caused by the higher biomass production and more intensive use of nutrients by resprouting hybrid aspen in the SRC site than by seedlings/saplings in the forestland.
4 Discussion
4.1 Impacts of above-ground biomass and stump harvesting in forestland
In the last few decades, the effects of intensified forest harvesting, including slash (small woody residues, branches and foliage) removal for bioenergy, on nutrient stores and cycling in forest ecosystems have frequently been discussed. Many studies have focused on changes in water chemistry and nutrient leaching after biomass harvesting, in particular on the differences between whole-tree and stem-only harvesting (Katzensteiner 2003; Wall 2008; Hedwall et al. 2013; Clarke et al. 2017; Libiete et al. 2017). During WTH, considerably more nutrients are exported from the site compared to SOH (Achat et al. 2015) because of the high concentrations of elements in the foliage and small branches (Neirynck et al. 1998); lower growth rates of several conifer species have been recorded on sites where WTH has been carried out (Proe et al. 1996; Egnell and Leijon 1999).
Nutrient uptake by the young forest generation is more intense compared to the compensatory processes, such as weathering, deposition and fixation. Therefore, there are concerns related to nutrient deficiency in high-yielding forest stands where WTH has been performed, as there is no resupply of base cations and nutrients (e.g. Akselsson et al. 2007). In our case, the Oxalidosa turf. mel. (Kudrenis) site would theoretically be subject to the highest risk of nutrient deficiency for the young forest stand; however, the increased supply of base cations with confined aquifer discharge may at least partly compensate for the increased mineralisation of organic matter. Confined aquifer discharge is an important factor influencing nutrient cycling in Latvian forests, as considerably more than half of all waterlogged and drained forests are located in confined aquifer discharge areas (Virbulis et al. 2013; Indriksons and Zalitis 2000; Zālītis 2012). Further investigations of soil and water processes and measurements of the young stand will reveal the potential differences between the treatments in the following years.
Elevated NO3–-N concentrations in the soil solution may indicate a potential risk of nutrient leaching, and clearfelling generally increases this risk (Nieminen 2004; Laurén et al. 2005; Gundersen et al. 2006). The nitrate nitrogen concentration in the soil solution is strongly affected by the slash biomass left on the forest floor, which may serve as a nutrient sink as well as a nutrient source while decomposing (Barber and Van Lear 1984; Strahm et al. 2005; Devine et al. 2012). On the other hand, leaching may be enhanced by the production of nitrate from decomposing logging residues (Staaf and Olsson 1994; Mahendrappa et al. 2006), and therefore, greater leaching may be expected from stands with stem-only harvest compared to whole-tree harvest. Furthermore, slashing interacts with the vegetation and affects the microclimate of the soil (Thiffault et al. 2011; Achat et al. 2015). In our study, on a nutrient-poor site (Myrtillosa (Zveri)), the gradual decomposition of logging residues has increased the soil solution NO3–-N concentration more in the SOH subplot than in the WTH subplot, and the same trend applies when NH4+-N and TN concentrations are analysed. In more fertile sites, Hylocomiosa (Vilkukalns) and, especially, Oxalidosa turf. mel. (Kudrenis) (drained peatland), the opposite trend was observed – higher NO3–-N, NH4+-N and TN concentrations were observed in the WTH plot.
While a previous study, using data from the years 2012–2014, revealed no statistically significant differences between harvesting regimes in the Hylocomiosa (Vilkukalns) site (Libiete et al. 2017), NO3–-N and TN concentrations in 2015–2017 were higher in the WTH subplot. A similar situation was observed in the Oxalidosa turf. mel. (Kudrenis) site, with the differences in NO3–-N and TN concentration between WTH and SOH levelling out in 2017–2018. In these sites, other factors, such a an already high soil N level, soil disturbance, N uptake capacity by ground vegetation, may have been of higher importance for the nitrogen concentration increase than the availability of organic matter in the form of logging residues. For example, Devine et al. (2012) found that N leaching after the harvest at high-productivity site was five times greater than at low-productivity sites, and soil N was likely the most important source. The same authors concluded that N leaching was higher after SOH than after WTH, but the influences varied considerably among sites.
It has been noted that deposition and weathering could not sustain sufficient nutrient stocks during WTH in the long term in nutrient-poor sites, but SOH treatment shows no indications of nutrient depletion for the following 10 rotation periods (Vangansbeke et al. 2015). Our results show a negligible difference between treatments, although a slightly higher risk of nutrient leaching was noticeable in the WTH subplot in the Hylocomiosa (Vilkukalns) site and the Oxalidosa turf. mel. (Kudrenis) site. Risks of nutrient leaching are related not only to the availability of organic matter for decomposition, but also to the site productivity, soil properties, vegetation, among others. Several authors stress the need for long-term studies of this subject and broader analyses of various factors influencing nutrient cycling. We strongly support this opinion and will expand the analyses in the following stages of the research.
Stump-harvest effects on soil water depend largely on harvest intensity, e.g. the amount of biomass harvested and the soil disturbance caused by the harvest operations. Several studies show increased nutrient (especially nitrate) leaching into groundwater and watercourses caused by stump harvesting (Egnell et al. 2007; Persson 2016; Magnusson 2017; Persson et al. 2017).
Two factors are likely to be responsible for the increased risk of nitrate leaching: (1) Fungi decomposing stumps and coarse roots can import ammonium and nitrate to the stump/root system from the surrounding soil, thus causing immobilisation of N compounds. When stumps are removed, mobile inorganic N is left in the soil and can be leached; (2) At stump harvesting, the soil is disturbed and mixed, whereby ammonium-oxidising microorganisms from deeper soil layers can be incorporated into soil layers with plenty of ammonium, which they convert to nitrate. This effect is similar to that of the site preparation, but the impact of stump harvesting may be deeper; moreover, as site preparation is usually carried out afterwards anyway, a larger proportion of the soil surface area is disturbed, with the mixing effect of stump harvesting likely to persist for a prolonged period of time (Kaarakka 2018). According to data presented by Kataja-aho et al. (2011), the coverage of the intact soil in the stump removal sites was more than than 20% less than in sites where no stump harvesting but only site preparation was carried out.
Both factors would result in increased risks of nitrate leaching, and in a field experiment in southern Sweden, increased nitrate leaching below the rooting zone has been documented (Persson and Olsson 2017). Staaf and Olsson (1994) concluded that stump and slash harvesting increased the ammonium concentration in the soil compared to just slash harvesting during the first 2 years, but during the third and fourth year, nitrate levels in the soil water increased dramatically. The concentrations in the plots with stump and slash harvest were, on average, five times higher than those in areas with just slash harvest, which corresponded to an outflow of 50 and 10 kg NO3–-N ha–1, respectively, in these 2 years. The most likely explanation for the higher nitrate leaching after stump harvesting is therefore the absence of N immobilisation from stumps/roots in combination with strong soil mixing (Persson and Olsson 2017). Our research sites did not demonstrate pronounced nitrogen concentration increases in the subplots where stump harvesting was performed, except for the Nitaure site in the fourth year after harvesting.
As one of the sites of above-ground biomass removal experiment and all sites of stump extraction experiment belong to the same site type (Hylocomiosa), it was, to some extent, possible to compare the impacts of three forest management regimes – stem-only harvesting, whole tree harvesting and whole-tree harvesting with stump removal – on the nitrogen concentrations (average of 5 years after harvesting). The lowest NO3–-N concentrations were found for plots with stump extraction, the average levels of the SOH plot exceeding these by eight times, but the average levels of WTH plots exceeding the values of WTH + SB up to 11 times (depending on the study site). The TN concentration differences followed a similar pattern, but the differences in the NH4+-N concentration between the management regimes were less explicit, with a maximum of three times between the WTH + SB and WTH variants. This can be explained by the lower availability of organic matter for the decomposing organisms in the plots where stump extraction has been carried out. Several studies suggest that the effect of stump removal on soil C and N pools is low compared to the effect of slash removal (Egnell 2016; Jurevics et al. 2016), as slash contains higher concentrations of nutrients than stumps and coarse roots (Iwald et al. 2013).
4.2 Impact of biomass harvesting in the SRC
Nitrogen sources in soils, released by mineralisation processes, are protected from N-leaching as long as the N uptake by the vegetation cover balances the N release. If plant N uptake s diminished for some reason – for instance, due to biomass harvesting, as was the case in our study – but mineralisation processes continue, nitrate leaching may occur. However, since harvesting is done normally during winter, when mineralisation rates are low and the rootstock immediately sprouts once the weather gets warmer, nitrification pulses after harvest have not been described so far, even in cases with additional N fertilisation (Schmidt-Walter and Lamersdorf 2012). Thus, harvesting operations only slightly affect nutrient concentrations in the soil solution, and intensive management of SRCs should not be considered a major threat to water quality (Aronsson et al. 2000; Goodlass et al. 2007). The results of our study show that above-ground biomass harvesting in 5-year-old hybrid aspen SRC only slightly affected NO3–-N and NH4+-N concentrations in the soil solution during the subsequent 3 years. Such results were expected, as also results from other countries give evidence on consistently reduced nutrient leaching in SRCs (Tully and Ryals 2017) due to intensive nutrient, particularly nitrate, uptake and cycling in such ecosystems (Meiresonne et al. 2006). Furthermore, the mean NO3–-N concentrations in the soil solution remained lower than the quality threshold value (10.3 mg NO3–-N l–1) stated in the national legislation, Water Framework Directive and Nitrate Directive. On the contrary, a significant biomass harvesting impact on the TN concentration in the soil solution (decrease of average values) was found in several subplots of the hybrid aspen SRC with additional N fertilisation.
Our research hypotheses were, however, only partly supported. In forestland sites of medium to high fertility, we found higher concentrations and more pronounced increases in N compounds in the soil solution in the WTH subplots compared to the SOH or control subplots, but the opposite trend was found for sites with lower fertility conditions. At the same time, we found no significant differences in the concentration of N compounds between both studied treatments (WTH and WTH + SB) in the stump harvesting sites, and no significant differences between harvested and control subplots were detected in the SRC site in agricultural land, except for some cases with TN concentrations that were random and likely dependent on local site conditions.
5 Conclusions
In above-ground biomass harvesting experimental sites, both the NO3–-N concentration in the soil solution and the differences from the unharvested control site gradually increased after clearfelling. However, 6 years after harvesting, nitrate-nitrogen levels approached the values detected in the control plots or even decreased below those.
In the stump removal experimental sites, nitrate-nitrogen concentrations after harvesting tended to decrease in both WTH + SB and WTH plots. Significant differences in nitrate concentrations between the variants were observed only randomly, without any clear trends.
Above-ground biomass harvesting in the 5-year-old hybrid aspen SRC only slightly affected NO3–-N and NH4+-N concentrations in the soil solution during the subsequent 3 years after harvesting. However, a significant biomass harvesting impact on TN concentration in the soil solution (decrease in average values) was found in several subplots of the hybrid aspen SRC with additional N fertilisation.
The risks of N leaching immediately after harvesting appeared to be higher in forestland than in the SRC established on agricultural land, but the mid-term and long-term effects remain to be further investigated.
Acknowledgements
The first and second experiments were conducted with financial and technical support from JSC ‘Latvia’s State Forests’ in the framework of the research programme ‘The impact of forest management on ecosystem services provided by forests and related ecosystems’ (Nr. 5-5.5_006_101_16_6), implemented by LSFRI ‘Silava’. Establishment of the hybrid aspen SRC experimental plot was supported by the European Regional Development Fund’s project ‘Elaboration of models for establishment and management of multifunctional plantations of short-rotation energy crops and deciduous trees’ (No. 2010/0268/2DP/2.1.1.2.0/10/APIA/VIAA/118), and the continuation of the research work was supported by the European Regional Development Fund’s project ‘Developing the methods of plantation cultivation of fast-growing forest crops and evaluating the suitability of their wood for pelletising’ (No. 2013/0049/2DP/2.1.1.10/13/APIA/VIAA/031).
References
Achat D.L., Deleuze C., Landmann G., Pousse N., Ranger J., Augusto L. (2015). Quantifying consequences of removing harvesting residues on forest soils and tree growth – a meta-analysis. Forest Ecology and Management 348: 124–141. https://doi.org/10.1016/j.foreco.2015.03.042.
Ahtiainen M. (1992). The effects of forest clear-cutting and scarification on the water quality of small brooks. Hydrobiologia 243(1): 465–473. https://doi.org/10.1007/BF00007064.
Akselsson C., Westling O., Sverdrup H., Holmqvist J., Thelin G., Uggla E., Malm G. (2007). Impact of harvest intensity on long-term base cation budgets in Swedish forest soils. Acid Rain - Deposition to Recovery: 201–210. https://doi.org/10.1007/978-1-4020-5885-1_22.
Aronsson P.G., Bergström L.F., Elowson S.N.E. (2000). Long-term influence of intensively cultured short-rotation willow coppice on nitrogen concentrations in groundwater. Australasian Journal of Environmental Management 58(2): 135–145. https://doi.org/10.1006/jema.1999.0319.
Barber B.L., Van Lear D.H. (1984). Weight loss and nutrient dynamics in decomposing woody loblolly pine logging slash. Soil Science Society of America Journal 48(4): 906–910. https://doi.org/10.2136/sssaj1984.03615995004800040041x.
Bardule A., Rancane S., Gutmane I., Berzins P., Stesele V., Lazdina D., Bardulis A. (2013). The effect of fertiliser type on hybrid aspen increment and seed yield of perennial grass cultivated in the agroforestry system. Agronomy Research 11(1): 13–25.
Bardule A., Lazdins A., Sarkanabols T., Lazdina D. (2016). Fertilized short rotation plantations of hybrid aspen (Populus tremuloides Michx. × Populus tremula L.) for energy wood or mitigation of GHG emissions. Engineering for Rural Development 2016: 248–255.
Bardule A., Grinfelde I., Lazdina D., Bardulis A. (2018). Macronutrient leaching in a fertilized juvenile hybrid aspen (Populus tremula L. × P. tremuloides Michx.) plantation cultivated in an agroforestry system in Latvia. Hydrology Research 49(2): 407–420. https://doi.org/10.2166/nh.2017.054.
Bishop K., Allan C., Bringmark L., Garcia E., Hellsten S., Högbom L., Johansson K., Lomander A., Meili M., Munthe J., Nilsson M., Porvari P., Skyllberg U., Sorensen R., Zetterberg T., Akerblom S. (2009). The effects of forestry on Hg bioaccumulation in nemoral/boreal waters and recommendations for good silvicultural practice. Ambio 38(7): 373–380. https://doi.org/10.1579/0044-7447-38.7.373.
Bušs K. (1981). Forest ecology and typology. ‘Zinātne’, Rīga, Latvia, 64 p. [In Latvian].
Calder I., Hofer T., Vermont S., Warren P. (2007). Towards a new understanding of forests and water. Unasylva 229(58): 1–8.
Clarke N., Skår S., Kjønaas O.J., Hanssen K.H., Økland T., Nordbakken J.F, Eldhuset T.D., Lange H. (2017). Effects of forest residue harvesting on short-term changes in soil solution chemistry. Scandinavian Journal of Forest Research 33(3): 299–307. https://doi.org/10.1080/02827581.2017.1375141.
Conley D.J. (2012). Save the Baltic Sea. Nature 486(7404): 463–464. https://doi.org/10.1038/486463a.
Devine W.D., Footen P.W., Strahm B.D., Harrison R.B., Terry T.A., Harrington T.B. (2012). Nitrogen leaching following whole-tree and bole-only harvests on two contrasting Pacific Northwest sites. Forest Ecology and Management 267: 7–17. https://doi.org/10.1016/j.foreco.2011.11.043.
Diaz-Pines E., Molina-Herrera S., Dannenmann M., Braun J., Haas E., Willibald G., Arias-Navarro C., Grote R., Wolf B., Saiz G., Aust C., Schitzler J.P., Butterbach-Bahl K. (2016). Nitrate leaching and soil nitrous oxide emissions diminish with time in a hybrid poplar short-rotationcoppice in southern Germany. GCB Bioenergy 9(3): 613–626. https://doi.org/10.1111/gcbb.12367.
Dimitriou I., Mola-Yudego B. (2017). Impact of Populus Plantations on Water and Soil Quality. Bioenergy Research 10(3): 750–759. https://doi.org/10.1007/s12155-017-9836-5.
Dimitriou I., Busch G., Jacobs S., Schmidt-Walter P., Lamersdorf N. (2009). A review of the impacts of Short Rotation Coppice cultivation on water issues. Landbauforschung – vTI, Agriculture and Forestry Research 3(59): 197–206.
Egnell G. (2016). Effects of slash and stump harvesting after final felling on stand and site productivity in Scots pine and Norway spruce. Forest Ecology and Management 371: 42–49. https://doi.org/10.1016/j.foreco.2016.03.006.
Egnell G., Hyvonen R., Högbom L., Jahansson T., Lundmark T., Olsson B., Ring E., von Sydow F. (2007). Environmental aspects on stump-harvest – compilation of knowledge and knowledge gaps. Energimyndigheten, Rapport ER 2007:40. 116 p. [In Swedish].
European Environment Agency (2017). Pressure from critical loads from nitrogen. https://www.eea.europa.eu/data-and-maps/figures/pressure-from-critical-loads-from-nitrogen. [Cited 11 Jun 2019].
European Parliament (2009). Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009. Official Journal of the European Union 140(16): 16–62. https://doi.org/10.3000/17252555.L_2009.140.eng.
European Parliament C. (2000). Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Official Journal of the European Parliament.
Goodlass G., Green M., Hilton B., McDonough S. (2007). Nitrate leaching from short-rotation coppice. Soil Use and Management 23(2): 178–184. https://doi.org/10.1111/j.1475-2743.2006.00080.x.
Guénon R., Bastien J.C., Thiébeau P., Bodineau G., Bertrand I. (2016). Carbon and nutrient dynamics in short-rotation coppice of poplar and willow in a converted marginal land, a case study in central France. Nutrient Cycling in Agroecosystems 106(3): 293–309. https://doi.org/10.1007/s10705-016-9805-y.
Gundersen P., Schmidt I.K., Raulund-Rasmussen K. (2006). Leaching of nitrate from temperate forests: effects of air pollution and forest management. Environmental Reviews 14(1): 1–57. https://doi.org/10.1139/a05-015.
GURINIMAS (2019). Comperative overview of reactive nitrogen (Nr) flowa in Latvia and Estonia. https://www.envir.ee/sites/default/files/comparative_overview_of_reactive_nitrogen_nr_flows_in_latvia_and_estonia_pdf.pdf. [Cited 11 Jun 2019].
Hartwich J., Bölscher J., Schulte A. (2016). The impact of short rotation coppice on water and land resources. In: Hartmann T., Spit T. (eds.). Frontiers of land and water governance in urban regions, the impact of short rotation coppice on water and land resources. Routledge.
Hedwall P.-O., Grip H., Linder S., Lövdahl L., Nilsson U., Bergh J. (2013). Effects of clear-cutting and slash removal on soil water chemistry and forest-floor vegetation in a nutrient optimised Norway spruce stand. Silva Fennica 47(2) article 933. https://doi.org/10.14214/sf.933.
HELCOM (2007). HELCOM Baltic Sea Action Plan. http://www.helcom.fi/Documents/Baltic%20sea%20action%20plan/BSAP_Final.pdf. [Cited 13 Nov 2017].
Högbom L., Futter M. (2013). Quantifying N, P and C losses to waters from Fennoscandic /Baltic forests and the effect of various forestry operations. http://nordicforestresearch.org/wp-content/uploads/2017/09/SNS-110_Policy-Brief.pdf. [Cited 13 Nov 2017].
Indriksons A., Zālītis P. (2000). The impact of hydrotechnical drainage on cycle of some biogenous elements in forest. Baltic Forestry 6(1): 18–24.
Iwald J., Löfgren S., Stendahl J., Karltun E. (2013). Acidifying effect of removal of tree stumps and logging residues as compared to atmospheric deposition. Forest Ecology and Management 290: 49–58. https://doi.org/10.1016/j.foreco.2012.06.022.
Jurevics A., Peichl M., Olsson B.A., Strömgren M., Egnell G. (2016). Slash and stump harvest have no general impact on soil and tree biomass C pools after 32–39 years. Forest Ecology and Management 371: 33–41. https://doi.org/10.1016/j.foreco.2016.01.008.
Kaarakka L. (2018). Soil changes and long-term ecosystem recovery from physical and chemical load – stump harvesting and sprinkling infiltration as case studies. Dissertationes Forestales 260. 62 p. https://doi.org/10.14214/df.260.
Kataja-aho S., Fritze H., Haimi J. (2011). Short-term responses of soil decomposer and plant communities to stump harvesting in boreal forests. Forest Ecology and Management 262: 379–388. https://doi.org/10.1016/j.foreco.2011.04.002.
Katzensteiner K. (2003). Effects of harvesting on nutrient leaching in a Norway spruce (Picea abies Karst.) ecosystem on a Lithic Leptosol in the Northern Limestone Alps. Plant and Soil 250(1): 59–73. https://doi.org/10.1023/A:1022821913932.
Keenan R.J., Kimmins P.J. (1993). The ecological effects of clear-cutting. Environmental Reviews 1(2):121–144. https://doi.org/10.1139/a93-010.
Latvian Ministry of Economics (2010). National Renewable Energy Action Plan (NREAP). https://ec.europa.eu/energy/en/topics/renewable-energy/national-renewable-energy-action-plans-2020.
Laurén A., Finér L., Koivusalo H., Kokkonen T., Karvonen T., Kellomäki S., Mannerkoski H., Ahtiainen M. (2005). Water and nitrogen processes along a typical water flowpath and streamwater exports from a forested catchment and changes after clear-cutting: a modelling study. Hydrology and Earth System Sciences Discussions, European Geosciences Union 9(6): 657–674. https://doi.org/10.5194/hess-9-657-2005.
Libiete Z., Bardule A., Murniece S., Lupikis A. (2017). Impact of clearfelling on dissolved nitrogen content in soil-, ground-, and surface waters: initial results from a study in Latvia. Agronomy Research 15(3): 767–787.
Magnusson T.P.T. (2017). Long-term effects of stump harvest on mercury and general chemistry in discharge water. Scandinavian Journal of Forest Research 32(3): 230–238. https://doi.org/10.1080/02827581.2016.1244287.
Mahendrappa M.K., Pitt C.M., Kingston D.G.O., Morehouse T. (2006). Environmental impacts of harvesting white spruce on Prince Edward Island. Biomass and Bioenergy 30(4): 363–369. https://doi.org/10.1016/j.biombioe.2005.07.016.
Meiresonne L., De Schrijver A., De Vos B. (2006). Nutrient cycling in a poplar plantation (Populus trichocarpa x Populus deltoïdes ‘Beaupré’) on former agricultural land in northern Belgium. Canadian Journal of Forest Research 37(1): 141–155. https://doi.org/10.1139/x06-205.
Neirynck J., Maddelein D., De Keersmaeker L., Lust N., Muys B. (1998). Biomass and nutrient cycling of a highly productive Corsican pine stand on former heathland in northern Belgium. Annales des Sciences Forestières 55(4): 389–405. https://doi.org/10.1051/forest:19980401.
Nieminen M. (2004). Export of dissolved organic carbon, nitrogen and phosphorus following clear-cutting of three Norway spruce forests growing on drained peatlands in southern Finland. Silva Fennica 38(2): 123–132. https://doi.org/10.14214/sf.422.
Persson T. (2016). Stump harvesting – impact on climate and environment. Forest Ecology and Management 371: 1–4. https://doi.org/10.1016/j.foreco.2016.04.046.
Persson T., Olsson B.A. (2017). N mineralization and N leaching after stump harvesrting. In: Persson T., Egnell G., Lithell C. (eds.). Stump harvesting impact on climate and environment. IEA Bioenergy Task 43 TR2017:02.
Persson T., Lenoir L., Vegerfors B. (2017). Long-term effects of stump harvesting and site preparation on pools and fluxes of soil carbon and nitrogen in central. Scandinavian Journal of Forest Research 32(3): 222–229. https://doi.org/10.1080/02827581.2016.1218043.
Proe M.F., Cameron A.D., Dutch J., Christodoulou X.C. (1996). The effect of whole-tree harvesting on the growth of second rotation Sitka spruce. Forestry 69(4): 389–401. https://doi.org/10.1093/forestry/69.4.389.
Rönnberg C., Bonsdorff E. (2004). Baltic Sea eutrophication: area-specific ecological consequences. In: Kautsky H., Snoeijs P. (eds.). Biology of the Baltic Sea. Developments in Hydrobiology. Springer, Dordrecht, Netherlands. p. 227–241.
Schmidt-Walter P., Lamersdorf N.P. (2012). Biomass production with willow and poplar short rotation coppices on sensitive areas – the impact on nitrate leaching and groundwater recharge in a drinking water catchment near Hanover, Germany. BioEnergy Research 5(3): 546–562. https://doi.org/10.1007/s12155-012-9237-8.
Staaf H., Olsson B.A. (1994). Effects of slash removal and stump harvesting on soil water chemistry in a clear-cutting in SW Sweden. Scandinavian Journal of Forest Research 9(1–4): 305–310. https://doi.org/10.1080/02827589409382844.
Stålnacke P. (1996). Nutrient loads to the Baltic Sea. PhD thesis. Linköping Studies in Arts and Science 146. 78 p.
Stålnacke P., Aakerøy P.A., Blicher-Mathiesen G., Iital A., Jansons V., Koskiaho J., Kyllmar K., Lagzdins A., Pengerud A., Povilaitis A. (2014). Temporal trends in nitrogen concentrations and losses from agricultural catchments in the Nordic and Baltic countries. Agriculture, Ecosystems & Environment 198: 94–103. https://doi.org/10.1016/j.agee.2014.03.028.
Strahm B.D., Harrison R.B., Terry T.A., Flaming B.L., Licata C.W., Petersen K.S. (2005). Soil solution nitrogen concentrations and leaching rates as influenced by organic matter retention on a highly productive Douglas-fir site. Forest Ecology and Management 218(1–4): 74–88. https://doi.org/10.1016/j.foreco.2005.07.013.
Thiffault E., Hannam K.D., Paré D., Titus B.D., Hazlett P.W., Maynard D.G., Brais S. (2011). Effects of forest biomass harvesting on soil productivity in boreal and temperate forests — a review. Environmental Reviews 19(1): 278–309. https://doi.org/10.1139/a11-009.
Tully K., Ryals R. (2017). Nutrient cycling in agroecosystems: balancing food and environmental objectives. Agroecology and Sustainable Food Systems 41(7): 761–798. https://doi.org/10.1080/21683565.2017.1336149.
Vangansbeke P., De Schrijver A., De Frenne P., Verstraeten A., Gorissen L., Verheyen K. (2015). Strong negative impacts of whole tree harvesting in pine stands on poor, sandy soils: a long-term nutrient budget modelling approach. Forest Ecology and Management 356: 101–111. https://doi.org/10.1016/j.foreco.2015.07.028.
Virbulis J., Bethers U., Saks T., Sennikovs J., Timuhins A. (2013). Hydrogeological model of the Baltic Artesian Basin. Hydrogeology Journal 21(4): 845–862. https://doi.org/10.1007/s10040-013-0970-7.
Wall A. (2008). Effect of removal of logging residue on nutrient leaching and nutrient pools in the soil after clearcutting in a Norway spruce stand. Forest Ecology and Management 256(6): 1372–1383. https://doi.org/10.1016/j.foreco.2008.06.044.
Zālītis P. (2012). Forest and water. Latvian State Forest Research Institute ‘Silava’, Salaspils, Latvia. 356 p. [In Latvian].
Total of 61 references.

