Sphaeropsis sapinea found as symptomless endophyte in Finland
Terhonen E.-L., Babalola J., Kasanen R., Jalkanen R., Blumenstein K. (2021). Sphaeropsis sapinea found as symptomless endophyte in Finland. Silva Fennica vol. 55 no. 1 article id 10420. https://doi.org/10.14214/sf.10420
Highlights
- Sphaeropsis sapinea was found for the first time as an endophyte in healthy Scots pine in Finland
- This finding confirms that S. sapinea can proliferate in a symptomless stage in Scots pine in Finland.
Abstract
The aim of this study was to determine if the ascomycete fungus Sphaeropsis sapinea (Fr.) Dyko & B. Sutton (syn. Diplodia sapinea (Fr.) Fuckel) could be cultured from surface sterilized Scots pine twigs presenting the endophytic stage of this fungus. This fungus causes the disease called Diplodia tip blight in conifers. Symptoms become visible when trees have been weakened by abiotic stressors related to temperature, drought and hailstorms. The disease is rapidly increasing and is observed regularly in Scots pine (Pinus sylvestris L.) forests in Europe. Changes in climatic conditions will gradually increase the damage of this pathogen, because it is favored by elevated temperatures and additionally the host trees will be more susceptible due to related environmental stress. Diplodia tip blight is emerging towards Northern latitudes, thus, actions to monitor the spread of S. sapinea in pine-dominated forests should be undertaken in Finland. Our aim was to search for S. sapinea in Scots pine along a transect in Finland. Branch samples were collected from healthy Scots pine, fungal endophytes were isolated and morphologically identified. Sixteen S. sapinea strains were found from four Scots pine trees from two locations. This finding confirms that S. sapinea is found as an endophyte in healthy Scots pine in Finland.
Keywords
Pinus sylvestris;
Scots pine;
Diplodia sapinea;
Diplodia tip blight
-
Terhonen,
Forest Pathology Research Group, Department of Forest Botany and Tree Physiology, Faculty of Forest Sciences and Forest Ecology, University of Goettingen, Büsgen-Institute, Büsgenweg 2, D-37077 Göttingen, Germany
E-mail
terhonen@uni-goettingen.de

- Babalola, Forest Pathology Research Group, Department of Forest Botany and Tree Physiology, Faculty of Forest Sciences and Forest Ecology, University of Goettingen, Büsgen-Institute, Büsgenweg 2, D-37077 Göttingen, Germany E-mail j.babalola@stud.uni-goettingen.de
- Kasanen, Forest Pathology Lab, Department of Forest Sciences, University of Helsinki, Latokartanonkaari 7, FI-00014 University of Helsinki, Finland E-mail risto.kasanen@helsinki.fi
- Jalkanen, Rovaniemi Research Unit, Natural Resources Institute Finland (Luke), Eteläranta 55, FI-96300 Rovaniemi, Finland E-mail ristjal@gmail.com
- Blumenstein, Forest Pathology Research Group, Department of Forest Botany and Tree Physiology, Faculty of Forest Sciences and Forest Ecology, University of Goettingen, Büsgen-Institute, Büsgenweg 2, D-37077 Göttingen, Germany E-mail kathrin.blumenstein@uni-goettingen.de
Received 24 July 2020 Accepted 16 December 2020 Published 26 January 2021
Views 93880
Available at https://doi.org/10.14214/sf.10420 | Download PDF

1 Introduction
Scots pine (Pinus sylvestris L.) is the main source of forest tree products for the forest sector in Finland contributing several billion EUR net yearly (Finnish Forest Statistics 2019). The major threats to the sustainable supply of forest tree products are adverse climate, pests and diseases. Damages due to fungal pathogens are predicted to increase under climate change scenarios that include increasing temperatures and longer periods of drought (Seidl et al. 2017). The forest pathosystems’ behavior can be unpredictable in the future due to changes in environment that favor fungal pathogens rather than the host (Linnakoski et al. 2017; Terhonen et al. 2019; Stewart et al. 2020). When the local environment changes and an increase in drought/temperature-associated stress may be observed, Scots pines become more susceptible to pathogens (Stanosz et al. 2001; Bußkamp 2018). Southern Finland is a high-risk drought area (Veijalainen et al. 2019), where a dry growing season may result in a 30–50% probability of detecting physiological changes such as defoliation, decreased carbon and nutrient assimilation, and breakdown of the photosynthetic process through loss of hydraulic conductivity in forest trees (Muukkonen et al. 2015).
Endophytes are described as microbes that live asymptomatically in their host-plant tissues for the entire or at least a significant part of their life cycle, without causing any visible negative symptoms to the host (Petrini 1991; Saikkonen et al. 1998). In practical applications (e.g. when isolating endophytes), the definition of endophytes is stated by Hallmann et al. (1997) as “…those that can be isolated from surface-disinfested plant tissue or extracted from within the plant, and that do not visibly harm the plant.” Plant pathogens and saprophytes can be endophytically present in host tissues and when the conditions become favourable for them (e.g. dead needle tissue is generated) they change from endophyte to pathogenic/saprophytic (Müller et al. 2001; Parfitt et al. 2010; Álvarez-Loayza et al. 2011). The endophytic stage is the typical strategy for shoot pathogens that exploit periods of abiotic stressors in the host (Slippers and Wingfield 2007; Blumenstein et al. 2020; Oliva et al. 2020). Sphaeoropsis sapinea (Fr.) Dyko & B. Sutton (syn. Diplodia sapinea (Fr.) Fuckel) is an ascomycete fungus with different trophic stages (Smith et al. 1996). It can live asymptomatically as an endophyte in its host tree (Langer et al. 2011; Luchi et al. 2014; Blumenstein et al. 2020; Bußkamp et al. 2020), while being a latent and opportunistic pathogen (Brodde et al. 2019; Blumenstein et al. 2020; Bußkamp et al. 2020) or/and saprophyte (Müller et al. 2019; Oliva et al. 2020). The abundance of S. sapinea is currently increasing in European forests as an endophyte (Bußkamp 2018; Blumenstein et al. 2020; Bußkamp et al. 2020; Oliva et al. 2020) and it is emerging in Northern latitudes (Hanso and Drenkhan 2007; Adamson et al. 2015; Brodde et al. 2019; Müller et al. 2019). Sphaeropsis sapinea causes the disease called Diplodia (syn.Sphaeropsis) tip blight and the outbreaks in Europe threaten the ecologically important and commercially valuable Scots pine forests (Fabre et al. 2011; Langer et al. 2011; Brodde et al. 2019; Blumenstein et al. 2020; Oliva et al. 2020).
Sphaeropsis sapinea spreads through conidial dispersion, transmitted by wind or water droplets (Brookhouser and Peterson 1971; Swart et al. 1987; Brodde et al. 2019). Seedlings can also transmit S. sapinea as asymptomatic nursery stock when the fungus is present as an endophyte (Stanosz et al. 2007). As for most tree endophytes, S. sapinea is transmitted horizontally (Rodriguez et al. 2009; Bihon et al. 2011). Fruiting bodies (pycnidia) are produced on dead twigs, needles or cones (Fig. 1A–B) and the asexual spores are released into the air from spring to fall (Brodde et al. 2019). Conidia are oval with a size of 30–55 × 11–18 µm (Sutton 1980; Sutton and Dyko 1989; Fig. 1C). Unwounded healthy tissue may become infected in spring/early summer during bud burst and tissue elongation (Brookhouser and Peterson 1971; Chou 1976, 1978). Similarly, S. sapinea also enters unwounded twig tissues through needle stomata (Brookhouser and Peterson 1971; Chou 1976; Flowers et al. 2006; Schlößer 2020). In addition, S. sapinea enters pine trees via injured tissue (Munck et al. 2009; Oliva et al. 2020), grow and persist for long time periods in young and older twig tissues without causing the disease (Bihon et al. 2011). When the environment changes towards conditions more adverse for the host tree (e.g. drought) and more favorable for pathogenic fungi (e.g. elevated temperature), Sphaeropsis sapinea switches from the endophytic stage to pathogenic (Fabre et al. 2011; Bosso et al. 2017; Bußkamp et al. 2020). After this lifestyle change, Sphaeropsis sapinea kills the twigs, and eventually it can kill even mature trees during one growth season (Blumenstein et al. 2020; Bußkamp et al. 2020; Oliva et al. 2020). Diplodia tip blight disease symptoms include tip blight, stem canker, dieback of current year shoots, and blue staining of the sapwood (Luchi et al. 2014; Bußkamp et al. 2020; Oliva et al. 2020). The lifestyle change can lead to fast developing epidemics in Scots pine-dominated forests (Bußkamp 2018; Brodde et al. 2019; Blumenstein et al. 2020). The disease, Diplodia tip blight, has increased rapidly but still the invasion and epidemiology studies are lacking (Flowers et al. 2006; Bußkamp 2018; Brodde et al. 2019; CABI 2019).

Fig. 1. Pycnidia of Sphaeropsis sapinea on (A) a Scots pine twig and (B) a needle. (C) Conidia of Diplodia sapinea released from a pycnidium (Ø 250 µm, modified from Schlößer 2020).
Desprez-Loustau et al. (2006) classify S. sapinea as cryptogenic. Cryptogenic species are defined as species with an uncertain origin, but that are suspected to be exotic (invasive). Similarly, in the “Handbook of alien species in Europe”, S. sapinea is classified as cryptogenic (DAISIE 2009). Although the origin of S. sapinea is still unknown, it can be introduced to new regions in host material such as cones, seeds, and diseased seedlings (Stanosz et al. 2007; Brodde et al. 2019; Cleary et al. 2019). It has been considered that S. sapinea could be an invasive species in Europe, or that it has changed its behavior in the last decades from an asymptomatic endophyte to a pathogen due to the change in environmental conditions (Stanosz et al. 2001; Bußkamp 2018; Brodde et al. 2019; Müller et al. 2019; Blumenstein et al. 2020). However, the invasive nature of S. sapinea in Northern Europe has not been confirmed. Sphaeropsis sapinea was recorded to cause disease symptoms for the first time in Europe in the early 1980s (van Dam and de Kam 1984) and in the mid-1990s (Heydeck and Dahms 2012), respectively. Other reports from Central-Europe indicate emergence of this opportunistic pathogen (Bußkamp 2018; Brodde et al. 2019; Bußkamp et al. 2020; Blumenstein et al. 2020; Oliva et al. 2020). Sphaeropsis sapinea was observed in Estonia in 2007 (Hanso and Drenkhan 2009) and in Sweden in 2013 (Oliva et al. 2013). In southern Finland it was found on cones (living as a saprophyte) in 2015 and in 2016 (Müller et al. 2019). The disease Diplodia tip blight caused by S. sapinea is emerging in the northern parts of Europe and poses a serious threat to pine-based silviculture in the Nordic and Baltic countries. Our aim was to determine if S. sapinea could be found as an endophyte (as defined by Petrini 1991) in symptomless Scots pine trees in Finland.
2 Materials and methods
2.1 Study sites and trees
Tips of branches from 80 Scots pine trees were randomly collected at fourteen sites in various parts of Finland during the summer of 2019 (Table 1). The site number three (3) is described in Müller et al. (2019). The collected branches included annual shoots of 2017, 2018, and 2019. Shoots were sampled at various heights of the trees of various ages (Table 1). Samples from Kivalo (site 13) were collected from several heights altogether from six trees (tree 1: 17 m, 19 m and 21 m; tree 2: 11 m, 13 m, 15 m, 17 m and 19 m; tree 3: 9 m, 11 m and 15 m; tree 4: 4 m and 8 m; tree 5: 8 m, 10 m and 12 m; tree 6: 11 m and 17 m).
| Table 1. Investigated Scots pine stands and number of collected samples. | ||||||||
| Site No. | Location | Latitude, Longitude | No. of trees | No. of branches | No. of tips | Avg. age of trees | Sampling month | Sampling height, m |
| 1 | Vantaa | 60°25´N, 25°07´E | 10 | 10 | 30 | 40 | July | 2 |
| 2 | Mäntsälä | 60°68´N, 25°19´E | 1 | 1 | 3 | 20 | July | 10 |
| 3 | Lohja | 60°29´N, 23°55´E | 10 | 10 | 30 | 20 | July | 2 |
| 4 | Lapinjärvi | 60°64´N, 26°15´E | 6 | 6 | 18 | 60 | July | 10 |
| 5 | Akaa | 61°16´N, 23°90´E | 11 | 11 | 33 | 40 | July | 2 |
| 6 | Urjala | 61°10´N, 23°52´E | 4 | 4 | 12 | 40 | July | 2 |
| 7 | Hyytiälä | 61°80´N, 24°30´E | 6 | 6 | 18 | 40 | July | 4 |
| 8 | Route 66 | 61°80´N, 24°30´E | 3 | 3 | 9 | 20 | July | 4 |
| 9 | Setälänkangas | 61°80´N, 24°20´E | 3 | 3 | 9 | 20 | July | 2 |
| 10 | Setälänkangas | 61°80´N, 24°20´E | 4 | 4 | 12 | 40 | July | 4 |
| 11 | Siikakangas | 61°90´N, 24°20´E | 5 | 5 | 15 | 40 | July | 4 |
| 12 | Skaftung | 62°15´N, 21°33´E | 8 | 8 | 24 | 40 | August | 2 |
| 13 | Kivalo | 66°00´N, 25°50´E | 6 | 6 | 18 | 80 and 40 | July | several |
| 14 | Lapajärvi | 66°70´N, 28°40´E | 3 | 3 | 9 | 35 | August | 10 |
| SUM: | 80 | 80 | 240 | |||||
2.2 Fungal isolation
Altogether one branch of each tree, presenting altogether 240 twigs, were sampled (240 twigs from 80 trees; each twig either 2017, 2018 or 2019 growth). The needles were removed from each sample, and 3 cm sections cut from the tip of each annual shoot were sterilized. The sections were dipped in 70% ethanol for 1 min, followed by surface sterilization in 2% NaOCl for 1 min, they were rinsed four times with autoclaved deionized water. The sections were then cut in 0.5 cm long pieces and individually placed on Petri plates containing 1.5 % malt extract agar (MEA). The plates were incubated at room temperature for 3 to 4 weeks to reveal the slow growing endophytes. The plates were sub-cultured until pure colonies were obtained. The plates were examined and the colonies with S. sapinea morphology (Fig. 2) were selected for molecular identification.

Fig. 2. Three examples of Sphaeropsis sapinea morphology on 1.5% MEA plate. The hyphae color can vary from light grey to intense black.
2.3 DNA extraction and PCR
Seven S. sapinea strains provided by Dr. Michael Müller (Müller et al. 2019) were included in DNA analysis in this study to confirm the taxonomy of new strains. Fungal DNA was extracted for the purpose of molecular identification following the protocol of Keriö et al. (2020). Briefly, 1000 µl of PVP extraction buffer (1M NaCl, 100 mM TrisHcl, 10 mM EDTA, 2% PVP (w/v)) was added to a 1.5 ml Eppendorf tube with 0.3 g of grounded mycelium sample. After incubation at 65 °C for 15 minutes, the sample was centrifuged at 5000 rpm for 10 min. The supernatant was transferred into a 1.5 ml tube (ca 500 µl). One volume of SDS buffer (1% SDS (w/v), 0.5 M KCl) was added. The sample was vortexed for 20 seconds and centrifuged at 15 000 rpm for 10 min. The supernatant was transferred into a 1.5 ml tube (ca 700 µl). Isopropanol (0.85 volumes) was added and mixed by inversion for 20 seconds, followed by centrifugation for 10 min at 15 000 rpm. The supernatant was removed by pouring it away, and the pellet was washed with 200 µl of cold 70% ethanol. After centrifugation at 15 000 rpm for 5 min, ethanol was removed, and the pellet dried for 15 min at 65 °C. The pellet was re-suspended in nuclease-free water (50 µl).
Taq DNA polymerase (VWR) was used for PCR amplification of ITS regions with primer pair ITS1-F (White et al. 1990) and ITS4 (Gardes and Bruns 1993), for Large Sub Unit (LSU) region with primers nu-LSU-287-5’-mpnF (Nelsen et al. 2011) and LR 6R (Vilgalys and Hester 1990). Briefly, the PCR protocol was as follows: 1X PCR Buffer, 200 µM dNTP, 0.5 µM primer 1, 0.5 µM primer 2, 1.5M MgCl2, 100ng template DNA, 0.2 U/µl DNA polymerase; the reaction was adjusted to 25 µl with autoclaved MQ H2O. The PCR conditions used for ITS region were 94 °C for 3 min; 30 cycles of 94 °C for 30 s, 55 °C for 1 min, 72 °C for 1 min, and 72 °C for 10 min. For LSU region, the only difference was the annealing temperature, 48 °C.
Possible contaminations were determined with a negative control using sterile water as template in both PCR protocols. RedStain was used to confirm DNA amplicons on a 1.5% agarose gel, and the visual detection was made by ultraviolet transillumination. ITS and LSU region PCR products were purified and sequenced using the ITS4 and LR-6R primers at Microsynth SEQLAB (Germany). The DNA extraction, PCR and sequencing were successful for 10 strains found in this study and for all seven strains from Müller et al. (2019).
The quality of all the obtained FASTA files were checked before analysis. The ITS sequences were extracted with an open source software ITSx to extract the ITS1 and ITS2 subregions from the fungal ITS sequences (Bengtsson-Palme et al. 2013). The ITS1 and ITS2 sequences were used for BLASTN (Zhang et al. 2000) searches against GenBank/NCBI (Sayers et al. 2011) to provide taxonomic identification. Similarly, the sequences were blasted against ITS1, ITS2 and LSU regions of the S. sapinea strains found in Müller et al. (2019). The sequences obtained in this study and the ones from Müller et al. (2019) were aligned with MUSCLE (Edgar 2004), and a phylogenetic tree was generated in MEGA 5.01 (Tamura et al. 2011) using the Neighbour-joining (NJ) analysis with 1000 bootstrap replicates. The sequences of the isolates found in this study were deposited in GenBank with the following accession numbers: MT763348–MT763357 for ITS, and MT763358–MT763367 for LSU.
3 Results
Sphaeropsis sapinea was isolated from sixteen annual shoots harvested from four healthy symptomless Scots pine trees located in two different sites (Fig. 3, Table 2). They were considered as endophytic isolates as the hosts were healthy and the bark was intact. This means that the mycelium of the endophyte was already well established inside the host (under the bark) and it originated from surface sterilized plant tissue.
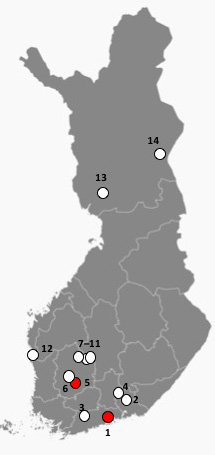
Fig. 3. Locations of the investigated Scots pine sites in this study as dots. Reprinted and modified from Free Vector Maps. Red dots indicate sites where Sphaeropsis sapinea was found in this study; number one (Vantaa) and five (Akaa).
| Table 2. Number and origin of Sphaeropsis sapinea isolates from healthy Scots pine shoots. | ||||||
| No. of S. sapinea isolates in annual shoots of Scots pine | ||||||
| No. of site | Location | No. of trees infected | Age of trees | 2019 | 2018 | 2017 |
| 1 | Vantaa | 2 | 40 | 5 | 4 | 3 |
| 5 | Akaa | 2 | 40 | 0 | 0 | 4 |
Morphological identification was performed for all 16 isolates (Fig. 2). Identification of S. sapinea was verified by ITS and LSU sequences for 10 isolates originating from the stands 1 (7 strains) and 5 (3 strains). ITS1 and ITS2 sequences were identical between strains and identical to numerous sequences in GenBank assigned to D. sapinea, or S. sapinea. They were also identical to those strains obtained recently from cones in Finland (Müller et al. 2019). LSU regions (for strains found in this study and those found in Müller et al. 2019) were identical (Query coverage 99%–100%; Per. Identity 98%–100%) to TYPE material of D. sapinea (gene bank ID: NG_069010). Scots pine stands infested by S. sapinea were close to the southwestern coast of Finland (Fig. 3). None of the fourteen investigated stands showed typical disease symptoms caused by S. sapinea at the time of sampling.
4 Discussion
Sphaeropsis sapinea is present as a symptomless endophyte in Scots pine twigs (Langer et al. 2011; Fabre et al. 2011; Luchi et al. 2014; Blumenstein et al. 2020; Bußkamp et al. 2020; Oliva et al. 2020). Similarly, we found S. sapinea as a symptomless endophytic fungus in healthy Scots pine trees (as defined Petrini 1991 and Hallmann et al. 1997) in Finland. This confirms that S. sapinea is emerging horizontally and has an endophytic trophic level in the northern limits of its known distribution area. Sphaeropsis sapinea was found on Austrian pine (Pinus nigra J.F.Arnold) in 2007 (Hanso and Drenkhan 2009) and on Scots pine in 2012 (Adamson et al. 2015) in Estonia. In 2013 the first observations of the pathogen were found in Sweden and in northwestern Russia (~110 km from the Finnish border) (Oliva et al. 2013; Adamson et al. 2015). Similarly, Sphaeropsis sapinea was observed as a saprophyte on the cones in 2015 in South Finland (Müller et al. 2019). It is notable that S. sapinea was not observed in Finland when Müller et al. (2019) did preliminary inventories in 2004 (Müller et al. 2019). The information describing how and where S. sapinea spread to Finland is still missing. The endophytic stage of S. sapinea can be detrimental for its Scots pine host, as S. sapinea can switch to pathogenic stage when triggered by host stress, and kill the occupied twigs leading eventually to the death of mature trees (Blumenstein et al. 2020; Fig. 4).

Fig. 4. Example of mature Scots pine stand that died during growth season of 2019, due disease caused by Sphaeropsis sapinea. Scots pine trees were stressed due to high temperatures and drought during 2010 and 2018 that lead to Diplodia tip blight disease epidemic, Germany 2019 (A). Blue stain developes fast in dead trees that can be seen after felling, partly this blue stain is due to S. sapinea, Germany 2019 (B).
The endophytic stage represents a balanced interaction between the fungus and its host tree. When conifer trees are stressed due to changes in the environment such as drought, temperature increases or hailstorms, Sphaeropsis sapinea transforms from an asymptomatic to pathogenic fungus (Stanosz et al. 2007; Bußkamp 2018; Blumenstein et al. 2020; Oliva et al. 2020). However, the mechanisms underlying the appearance of S. sapinea as first an asymptomatic fungal colonizer that turns pathogenic, after stimulation by host stress, remains unknown. Two possible scenarios related to temperature and drought could affect this transition. Sphaeropsis sapinea spores germinate best at 25 °C (Jiangyan et al. 1999). Similarly, the optimum of hyphae growth of S. sapinea is at 25 °C, while for other Scots pine endophytes the optimum growth is at 20 °C (Bußkamp 2018). In Finland, the annual mean temperature in the years 1979–2018 increased in Southern (+1.9 °C) and Northern (+2.7 °C) areas in Finland (Räisänen 2019). The increase in temperature is expected to be between 2.5 °C and 5 °C by the year 2100 (Mikkonen et al. 2015). Drought is an important abiotic disturbance factor, as the susceptibility of pine trees to S. sapinea is strongly enhanced by water stress (Stanosz et al. 2001; Boland et al. 2004; Deprez-Loustau et al. 2006; Blaschke and Cech 2007; Sturrock et al. 2011; Bußkamp 2018). The average annual precipitation sums were observed to vary from 450 mm in Northern Lapland to 750 mm in Southern and Eastern Finland for 1981–2010 (Pirinen et al. 2012). Veijalainen et al. (2019) analyzed that drought risk due to climate change can increase in Southern and Central Finland. Especially the drought risk during summer and early autumn can escalate due to an increase in temperature and longer summer periods (Veijalainen et al. 2019). Additional stress in Scots pines due to higher temperatures and drought in most vulnerable areas in Finland could lead to epidemics caused by S. sapinea. Brodde et al. (2019) showed that the epidemics observed in Sweden started to culminate already 10 years beforehand (S. sapinea emerging as an endophyte). Similarly, Blumenstein et al. (2020) showed that S. sapinea is the most common endophyte in healthy and diseased Scots pine and the diseased trees can die fast in one dry summer season (Blumenstein et al. 2020). This scenario can happen in near future in Finland, and it could be possible that changes in local environment causing stress to Scots pines can trigger these epidemics.
To mitigate the impacts of environmental changes, it is essential to understand the factors that trigger the development of forest tree disease epidemics and what increase host susceptibility. To design effective, durable and environmentally friendly disease prevention we need to understand the detailed epidemiology of this emerging fungi as well as the co-evolution history with its host. At the moment we don’t know all the factors participating to the epidemiology of this fungi, e.g. what is needed for the activation of the pathogenic stage and how S. sapinea can emerge to new areas. This information can ultimately be used to improve forest health via resistance breeding, to discover biocontrol methods, and to develop diagnostic methods to limit/detect the spread of the endophytic S. sapinea. These facts highlight the urgent need to restrict the influence of S. sapinea and to develop new management protocols to secure the health of pine-dominated forests in Finland.
Acknowledgements
We thank Professor Jarkko Hantula, Natural Resources Institute Finland, for providing branch samples from one of the sites (number three) and we thank Dr. Michael Müller, Natural Resources Institute Finland, for providing the previously found saprophytic fungal strains. This study was funded by Faculty of Forest Sciences and Forest Ecology, University of Goettingen, Germany. We acknowledge support by the Open Access Publication Funds of the Goettingen University.
References
Adamson K, Klavina D, Drenkhan R, Gaitnieks T, Hanso M (2015) Diplodia sapinea is colonizing the native Scots pine (Pinus sylvestris) in the northern Baltics. Eur J Plant Pathol 143: 343–350. https://doi.org/10.1007/s10658-015-0686-8.
Álvarez-Loayza P, White JF Jr, Torres MS, Balslev H, Kristiansen T, Svenning J-C, Gill N (2011) Light converts endosymbiotic fungus to pathogen, influencing seedling survival and niche-space filling of a common tropical tree, Iriartea deltoidea. PLoS ONE 6, article id e16386. https://doi.org/10.1371/journal.pone.0016386.
Bengtsson‐Palme J, Ryberg M, Hartmann M, Branco S, Wang Z, Godhe A, De Wit P, Sánchez‐García M, Ebersberger I, de Sousa F, Amend A, Jumpponen A, Unterseher M, Kristiansson E, Abarenkov K, Bertrand YJK, Sanli K, Eriksson KM, Vik U, Veldre V, Nilsson RH (2013) Improved software detection and extraction of ITS1 and ITS 2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol Evol 4: 914–919. https://doi.org/10.1111/2041-210X.12073.
Bihon W, Slippers B, Burgess T, Wingfield MJ, Wingfield BD (2011) Sources of Diplodia pinea endophytic infections in Pinus patula and P. radiata seedlings in South Africa. For Pathol 41: 370–375. https://doi.org/10.1111/j.1439-0329.2010.00691.x.
Blaschke M, Cech TL (2007) Absterbende Weisskiefern – eine langfristige Folge des Trockenjahres 2003. [Dying Scots Pines – a long-term consequence from the dry year 2003]. Forstschutz aktuell 40: 32–34.
Blumenstein K, Langer G, Bußkamp J, Langer E, Terhonen E (2020) The opportunistic pathogen Sphaeropsis sapinea is found to be one of the most abundant fungi in symptomless and diseased Scots pine in Central-Europe. BMC Plant Biology. [Preprint]. https://doi.org/10.21203/rs.3.rs-48366/v1.
Boland GJ, Melzer MS, Hopkin A, Higgins V, Nassuth A (2004) Climate change and plant diseases in Ontario. Can J Plant Pathol 26: 335–350. https://doi.org/10.1080/07060660409507151.
Bosso L, Luchi N, Maresi G, Cristinzio G, Smeraldo S, Russo D (2017) Predicting current and future disease outbreaks of Diplodia sapinea shoot blight in Italy: species distribution models as a tool for forest management planning. Forest Ecol Manag 400: 655–664. https://doi.org/10.1016/j.foreco.2017.06.044.
Brodde L, Adamson K, Julio Camarero J, Castaño C, Drenkhan R, Lehtijärvi A, Luchi N, Migliorini D, Sánchez-Miranda Á, Stenlid J, Özdağ Ş, Oliva J (2019) Diplodia tip blight on its way to the North: drivers of disease emergence in Northern Europe. Front Plant Sci 9, article id 1818. https://doi.org/10.3389/fpls.2018.01818.
Brookhouser LW, Peterson GW (1971) Infection of Austrian, Scots, and Ponderosa pines by Diplodia pinea. Phytopathology 61, article id 409. https://doi.org/10.1094/Phyto-61-409.
Bußkamp J (2018) Schadenserhebung, Kartierung und Charakterisierung des “Diplodia-Triebsterbens“ der Kiefer, insbesondere des endophytischen Vorkommens in den klimasensiblen Räumen und Identifikation von den in Kiefer (Pinus sylvestris) vorkommenden Endophyten. [Damage assessment, mapping and characterization of “Diplodia Tip Blight” of pines, especially the endophytic presence in climate-sensitive areas and identification of pine endophytes]. PhD thesis. Universität Kassel, Kassel.
Bußkamp J, Langer GJ, Langer EJ (2020) Sphaeropsis sapinea and fungal endophyte diversity in twigs of Scots pine (Pinus sylvestris) in Germany. Mycol Prog 19: 985–999. https://doi.org/10.1007/s11557-020-01617-0.
CABI (2019) Sphaeropsis sapinea (Sphaeropsis blight). Invasive Species Compendium. https://www.cabi.org/isc/datasheet/19160. Accessed 13 November 2019.
Chou CKS (1976) A shoot dieback in Pinus radiata caused by Diplodia pinea. 1. Symptoms, disease development, and isolation of pathogen. N Z J For Sci 6: 72–79.
Chou CKS (1978) Penetration of young stems of Pinus radiata by Diplodia pinea. Physiol Plant Pathol 13: 189–192. https://doi.org/10.1016/0048-4059(78)90033-4.
Cleary M, Oskay F, Doğmuş HT, Lehtijärvi A, Woodward S, Vettraino AM (2019) Cryptic risks to forest biosecurity associated with the global movement of commercial seed. Forests 10: 459. https://doi.org/10.3390/f10050459.
Delivering Alien Invasive Species Inventories for Europe (DAISIE) (n.d.) European Environment Agency. https://www.eea.europa.eu/data-and-maps/data-providers-and-partners/delivering-alien-invasive-species-inventories. Accessed 5 June 2020.
Desprez-Loustau M-L, Marçais B, Nageleisen L-M, Piou D, Vannini A (2006) Interactive effects of drought and pathogens in forest trees. Ann For Sci 63: 597–612. https://doi.org/10.1051/forest:2006040.
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. https://doi.org/10.1093/nar/gkh340.
Fabre B, Piou D, Desprez-Loustau M-L, Marçais B (2011) Can the emergence of pine Diplodia shoot blight in France be explained by changes in pathogen pressure linked to climate change? Glob Chang Biol 17: 3218–3227. https://doi.org/10.1111/j.1365-2486.2011.02428.x.
Finnish Forest Statistics (2019) Peltola A, Ihalainen A, Mäki-Simola E, Sauvula-Seppälä T, Torvelainen J, Uotila E, Vaahtera E, Ylitalo E (eds) Luonnonvarakeskus, Helsinki, Finland. ISBN 978-952-326-856-2.
Flowers J, Hartman JR, Vaillancourt LJ (2006) Histology of Diplodia pinea in diseased and latently infected Pinus nigra shoots. For Pathol 36: 447–459. https://doi.org/10.1111/j.1439-0329.2006.00473.x.
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for higher fungi and basidiomycetes: application to identification of mycorrhizae and rusts. Mol Ecol 2: 113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x.
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43: 895–914. https://doi.org/10.1139/m97-131.
Hanso M, Drenkhan R (2009) Diplodia pinea is a new pathogen on Austrian pine (Pinus nigra) in Estonia. Plant Pathol 58: 797–797. https://doi.org/10.1111/j.1365-3059.2009.02082.x
Heydeck P, Dahms C (2012) Trieberkrankungen an Waldbäumen im Brennpunkt der forstlichen Phytopathologie. [Shoot diseases of forest trees in the focus of forest phytopathology]. Eberswalder Forstliche Schriftenreihe 49: 47–55.
Jiangyan C, Xiaodong S, Shuhua L, Guirong L, Guijun X (1999) Studies on the biology of Sphaeropsis sapinea on Pinus sylvestris var. mongolica. LiaoNing Forestry Science and Technology 4, article id 712.
Keriö S, Terhonen E, LeBoldus JM (2020) Safe DNA-extraction protocol suitable for studying tree-fungus interactions. Bio Protoc 10, article id e3634. https://doi.org/10.21769/BioProtoc.3634.
Langer G, Bressem U, Habermann M (2011) Diplodia-Triebsterben der Kiefer und endophytischer Nachweis des Erregers Sphaeropsis sapinea. [Diplodia Tip Blight of pines and the endophytic proof of the pathogen Sphaeropsis sapinea] AFZ-Der Wald 11: 28–31.
Linnakoski R, Forbes KM, Wingfield MJ, Pulkkinen P, Asiegbu FO (2017) Testing projected climate change conditions on the Endoconidiophora polonica / Norway spruce pathosystem shows fungal strain specific effects. Front Plant Sci 8, article id 883. https://doi.org/10.3389/fpls.2017.00883.
Luchi N, Oliveira Longa CM, Danti R, Capretti P, Maresi G (2014) Diplodia sapinea: the main fungal species involved in the colonization of pine shoots in Italy. For Pathol 44: 372–381. https://doi.org/10.1111/efp.12109.
Mikkonen S, Laine M, Mäkelä HM, Gregow H, Tuomenvirta H, Lahtinen M, Laaksonen A (2015) Trends in the average temperature in Finland, 1847–2013. Stoch Environ Res Risk Assess 29: 1521–1529. https://doi.org/10.1007/s00477-014-0992-2.
Munck IA, Smith DR, Sickley T, Stanosz GR (2009) Site-related influences on cone-borne inoculum and asymptomatic persistence of Diplodia shoot blight fungi on or in mature red pines. Forest Ecol Manag 257: 812–819. https://doi.org/10.1016/j.foreco.2008.10.023.
Muukkonen P, Nevalainen S, Lindgren M, Peltoniemi M (2015) Spatial occurrence of drought-associated damages in Finnish boreal forests: results from forest condition monitoring and GIS analysis. Boreal Environ Res 20: 172–180.
Müller CB, Krauss J (2005) Symbiosis between grasses and asexual fungal endophytes. Curr Opin Plant Biol 8: 450–456. https://doi.org/10.1016/j.pbi.2005.05.007.
Müller MM, Hantula J, Wingfield M, Drenkhan R (2019) Diplodia sapinea found on Scots pine in Finland. For Pathol 49, article id e12483. https://doi.org/10.1111/efp.12483.
Müller MM, Valjakka R, Suokko A, Hantula J (2001) Diversity of endophytic fungi of single Norway spruce needles and their role as pioneer decomposers. Mol Ecol 10: 1801–1810. https://doi.org/10.1046/j.1365-294X.2001.01304.x.
Nelsen MP, Lücking R, Mbatchou JS, Andrew CJ, Spielmann AA, Lumbsch HT (2011) New insights into relationships of lichen-forming Dothideomycetes. Fungal Divers 51: 155–162. https://doi.org/10.1007/s13225-011-0144-7.
Oliva J, Boberg J, Stenlid J (2013) First report of Sphaeropsis sapinea on Scots pine (Pinus sylvestris) and Austrian pine (P. nigra) in Sweden. New Dis Rep 27: 23–23. https://doi.org/10.5197/j.2044-0588.2013.027.023.
Oliva J, Ridley M, Redondo MA, Caballol M (2020) Competitive exclusion amongst endophytes determines shoot blight severity on pine. Funct Ecol. https://doi.org/10.1111/1365-2435.13692.
Parfitt D, Hunt J, Dockrell D, Rogers HJ, Boddy L (2010) Do all trees carry the seeds of their own destruction? PCR reveals numerous wood decay fungi latently present in sapwood of a wide range of angiosperm trees. Fungal Ecol 3: 338–346. https://doi.org/10.1016/j.funeco.2010.02.001.
Petrini O (1991) Fungal endophytes of tree leaves. In: Andrews JH, Hirano SS (eds) Microbial ecology of leaves. Brock/Springer Series in Contemporary Bioscience. Springer, New York, pp 179–197. https://doi.org/10.1007/978-1-4612-3168-4_9.
Pirinen P, Simola H, Aalto J, Kaukoranta JP, Karlsson P, Ruuhela R (2012) Climatological statistics of Finland 1981–2010. Finnish Meteorological Institute Reports 1: 1–96.
Rodriguez RJ, White Jr JF, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182: 314–330. https://doi.org/10.1111/j.1469-8137.2009.02773.x.
Räisänen J (2019) Effect of atmospheric circulation on recent temperature changes in Finland. Clim Dyn 53: 5675–5687. https://doi.org/10.1007/s00382-019-04890-2.
Saikkonen K, Faeth SH, Helander M, Sullivan TJ (1998) Fungal endophytes: a continuum of interactions with hostpPlants. Annu. Rev. Ecol. Syst. 29: 319–343. https://doi.org/10.1146/annurev.ecolsys.29.1.319.
Sayers EW, Barrett T, Benson DA, Bolton E, Bryant SH, Canese K, Chetvernin V, Church DM, DiCuccio M, Federhen S, Feolo M, Fingerman IM, Geer LY, Helmberg W, Kapustin Y, Landsman D, Lipman DJ, Lu Z, Madden TL, Madej T, Maglott DR, Marchler-Bauer A, Miller V, Mizrachi I, Ostell J, Panchenko A, Phan L, Pruitt KD, Schuler GD, Sequeira E, Sherry ST, Shumway M, Sirotkin K, Slotta D, Souvorov A, Starchenko G, Tatusova TA, Wagner L, Wang Y, Wilbur WJ, Yaschenko E, Ye J (2011) Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 39: D38–D51. https://doi.org/10.1093/nar/gkq1172.
Schlößer R (2020) Natural infection ways of Sphaeropsis sapinea on Pinus sylvestris, Master thesis. University of Göttingen, Germany.
Seidl R, Thom D, Kautz M, Martin-Benito D, Peltoniemi M, Vacchiano G, Wild J, Ascoli D, Petr M, Honkaniemi J, Lexer MJ, Trotsiuk V, Mairota P, Svoboda M, Fabrika M, Nagel TA, Reyer CPO (2017) Forest disturbances under climate change. Nat Clim Chang 7: 395–402. https://doi.org/10.1038/nclimate3303.
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Rev 21: 90–106. https://doi.org/10.1016/j.fbr.2007.06.002.
Smith H, Wingfield M, Crous P, Coutinho TA (1996) Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. S Afr J Bot 62: 86–8. https://doi.org/10.1016/S0254-6299(15)30596-2.
Stanosz GR, Blodgett JT, Smith DR, Kruger EL (2001) Water stress and Sphaeropsis sapinea as a latent pathogen of red pine seedlings. New Phytol 149: 531–538. https://doi.org/10.1046/j.1469-8137.2001.00052.x.
Stanosz GR, Smith DR, Leisso R (2007) Diplodia shoot blight and asymptomatic persistence of Diplodia pinea on or in stems of jack pine nursery seedlings. For Pathol 37: 145–154. https://doi.org/10.1111/j.1439-0329.2007.00487.x.
Stewart J, Kim M-S, Lalande B, Klopfenstein NB (2020) Discovering key relationships between forest disease//health and microbial communities. Proceedings of the 66th Western International Forest Disease Work Conference, 3–7 June 2019, Estes Park, Colorado. WIFDWC, pp 19–20.
Sturrock RN, Frankel SJ, Brown AV, Hennon PE, Kliejunas JT, Lewis KJ, Worrall JJ, Woods AJ (2011) Climate change and forest diseases. Plant Pathol 60: 133–149. https://doi.org/10.1111/j.1365-3059.2010.02406.x.
Sutton BC (1980) The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Commonwealth Mycological Institute, UK. ISBN 9780851984469.
Sutton BC, Dyko BJ (1989) Revision of Hendersonula. Mycol Res 93: 466–488. https://doi.org/10.1016/S0953-7562(89)80040-1.
Swart WJ, Wingfield MJ, Knox-Davies PS (1987) Conidial dispersal of Sphaeropsis sapinea in three climatic regions of South Africa. Plant Dis 71: 1038–1040. https://doi.org/10.1094/PD-71-1038.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739. https://doi.org/10.1093/molbev/msr121.
Terhonen E, Langer GJ, Bußkamp J, Rӑscuţoi DR, Blumenstein K (2019) Low water availability increases necrosis in Picea abies after artificial inoculation with fungal root rot pathogens Heterobasidion parviporum and Heterobasidion annosum. Forests 10, article id 55. https://doi.org/10.3390/f10010055.
van Dam BC, de Kam M (1984) Sphaeropsis sapinea (= Diplodia pinea), oorzaak van het afsterven van eindscheuten bij Pinus in Nederland = Sphaeropsis sapinea (= Diplodia pinea), cause of dieback of top shoots with Pinus in the Netherlands. Mededeling / Rijksinstituut voor onderzoek in de bos- en landschapsbouw “De Dorschkamp”, article id 212.
Veijalainen N, Ahopelto L, Marttunen M, Jääskeläinen J, Britschgi R, Orvomaa M, Belinskij A, Keskinen M (2019) Severe drought in Finland: modeling effects on water resources and assessing climate change impacts. Sustainability 11: 2450. https://doi.org/10.3390/su11082450.
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172: 4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990.
White T, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal-RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, pp 315–322. https://doi.org/10.1016/b978-0-12-372180-8.50042-1.
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7: 203–214. https://doi.org/10.1089/10665270050081478.
Zwolinski JB, Swart WJ, Wingfield MJ (1995) Association of Sphaeropsis sapinea with insect infestation following hail damage of Pinus radiata. Forest Ecol Manag 72: 293–298. https://doi.org/10.1016/0378-1127(94)03459-A.
Total of 66 references.

