Recovery of boreal forest soil and tree stand characteristics a century after intensive slash-and-burn cultivation
Čugunovs M., Tuittila E.-S., Sara-Aho I., Pekkola L., Kouki J. (2017). Recovery of boreal forest soil and tree stand characteristics a century after intensive slash-and-burn cultivation. Silva Fennica vol. 51 no. 5 article id 7723. https://doi.org/10.14214/sf.7723
Highlights
- Soil organic matter stocks have still not fully recovered after a century of stand succession and passive recovery after slash-and-burn period
- Historical slash-and-burn stands feature higher live birch and standing dead wood volume than controls
- If passive rewildening is used, Fennoscandian boreal forests need more than a century to regain naturalness.
Abstract
Passive rewildening of forest ecosystems is commonly used for rehabilitating degraded habitats closer to their natural origin in addition to costly active restoration measures. However, it is not clear if passive processes are effective and how long the recovery of main ecosystem properties takes. We investigate the recovery of forest soil and tree stand characteristics a century after cessation of slash-and-burn cultivation, a major historical intensive disturbance regime that was applied widely in boreal zone of Finland until late 1800s. We systematically sampled soil and tree stand parameters within former slash-and-burn and nearby control areas. Humus layer thickness and soil organic matter (SOM) stocks were still lower in the historical slash-and-burn than in control areas. Slash-and-burn areas also had a larger volume of live birch trees and a higher standing dead wood volume than control areas. Accordingly, organic matter (humus layer thickness and SOM stocks) correlated negatively with birch standing live tree volume. Combined OM stock in humus and uppermost 10 cm mineral soil layer was positively correlated with lying dead wood volume. Overall, we observed clear recovery of several natural properties but we also found that a century after cessation of frequent anthropogenic burnings, clear legacies of disturbance in the above- and below-ground parts of boreal ecosystem were evident. Our results indicate that if only passive rewildening is applied as a restoration measure, the full recovery of boreal forest is slow and the effects of historical land-use may persist for over hundred years in soil and tree properties.
Keywords
restoration;
fire disturbance;
historical land-use;
rewildening
-
Čugunovs,
University of Eastern Finland, School of Forest Sciences, Yliopistokatu 7, P.O. Box 111, FI-80101 Joensuu, Finland
E-mail
mihails.cugunovs@uef.fi

-
Tuittila,
University of Eastern Finland, School of Forest Sciences, Yliopistokatu 7, P.O. Box 111, FI-80101 Joensuu, Finland
 http://orcid.org/0000-0001-8861-3167
E-mail
eeva-stiina.tuittila@uef.fi
http://orcid.org/0000-0001-8861-3167
E-mail
eeva-stiina.tuittila@uef.fi
- Sara-Aho, University of Eastern Finland, School of Forest Sciences, Yliopistokatu 7, P.O. Box 111, FI-80101 Joensuu, Finland E-mail ida.sara-aho@mhy.fi
- Pekkola, University of Eastern Finland, School of Forest Sciences, Yliopistokatu 7, P.O. Box 111, FI-80101 Joensuu, Finland E-mail laura.pekkola@gmail.com
-
Kouki,
University of Eastern Finland, School of Forest Sciences, Yliopistokatu 7, P.O. Box 111, FI-80101 Joensuu, Finland
 http://orcid.org/0000-0003-2624-8592
E-mail
jari.kouki@uef.fi
http://orcid.org/0000-0003-2624-8592
E-mail
jari.kouki@uef.fi
Received 10 May 2017 Accepted 1 November 2017 Published 10 November 2017
Views 152224
Available at https://doi.org/10.14214/sf.7723 | Download PDF

Supplementary Files
1 Introduction
Restoration has recently become an important approach to safeguard biodiversity (Similä and Junninen 2012; Halme et al. 2013). For example, in the Aichi targets under Convention on Biological Diversity (UN environment 2017) restoration is among the main activities to reach the global biodiversity goals. However, it is far from clear how disturbed ecosystems can be most efficiently restored. One possibility is to actively intervene and carry out specific manipulations in the ecosystems with the aim of restoring them to more natural states and functioning. For example, the use of restoration burnings is currently applied in Fennoscandian boreal forests (Hekkala et al. 2014; Kouki 2016). These burnings are typically of low to moderate severity (Čugunovs et al. 2017) and are to be repeated relatively rarely.
Another option is to let the ecosystems return to their “natural”, pre-disturbance state without active management. However, it is not clear if and how rapidly different ecosystems can recover, after just letting them to develop without direct human influence. The recovery is clearly related to the intensity of past land-use and the resilience of any ecosystem, a characteristic that is likely to vary depending on the regions, ecosystems and past disturbances (Holling 1996). It is also clear that many ecosystems have been altered in some direct or indirect ways by human activity, so the term “naturalness” becomes a relative one, and the reference baseline ecosystem state is often not completely natural or free of human impact legacies. This relativity of naturalness has been documented also for the boreal forests of eastern Fennoscandia (Uotila et al. 2002). Ecosystems retain human land-use legacies for decades and even centuries (Foster et al. 2003; Josefsson et al. 2009; Bürgi et al. 2017).
Recently, a land change science has emerged as an interdisciplinary field that aims to understand the dynamics of land cover and land-use in an era of global environmental change driven by interaction of human and environmental processes (Turner II et al. 2007).
In this context, abandonment of historical land-uses can provide opportunities to gain long-term perspectives (i.e. >100 years) on natural ecosystem recovery. In boreal forests, there is unexpected recent evidence that natural recovery following cessation of active land-use can in the long term lead to less diverse ecosystems (Oldén et al. 2017), but the authors suggest that on the landscape scale biodiversity nonetheless increases with passive recovery (Oldén et al. 2017). Other studies show that recovery in disturbance-driven habitats may lead toward the targeted undisturbed ecosystem (Nikodemus et al. 2012; Johnson et al. 2014; Tikkanen et al. 2014). However, so far the evidence is very limited and, for example, the role of fire and especially the long-term consequences of historically widely applied (Heikinheimo 1915; Lehtonen and Huttunen 1997) slash-and-burn cultivation on forest properties and recovery are poorly understood.
Slash-and-burn cultivation was practiced in Finland at least as early as in the Middle Ages, with the peak of use in the 1600–1800’s, and last burnings took place in early 20th century (Heikinheimo 1915; Lovén and Äänismää 2006). There were different variations of slash-and-burn practice depending on forest type, historical period and previous slash-and-burn activity locally. The most common measures involved, however, the felling of trees, burning them after a drying period, and oftentimes active soil preparation by harrowing before, during and after a burn (Lovén and Äänismää 2006). In contrast to slash-and-burn, contemporary forest burnings in boreal Fennoscandia can be an intentional method of restoration (Vanha-Majamaa et al. 2007; Kouki et al. 2012; Hekkala et al. 2014; Kouki 2016; Čugunovs et al. 2017) at low to moderate severities and frequencies, and with careful planning. Severe and frequent historical slash-and-burn disturbance regimes, however, can be considered a detrimental human-caused perturbation from which the forests may still need to recover. Such fire disturbance is expected to thin soil hemic and sapric i.e. Oe/Oa horizon (humus layer) and increase the prevalence of tree species typical to early stages of stand succession due to altered seedbed and shading conditions.
In this study, we analyze soil and tree stand characteristics in forests approximately 100 years after the cessation of intensive and several centuries long slash-and-burn cultivation period. The stands are compared to nearby old-growth forests that are known to have been outside of slash-and-burn practice in the observable past. By comparing stands with different fire histories and human influence, we aim to assess if these previously disturbed forests are now approaching natural-like condition, and what specific characteristics may still show the effect of past management. We hypothesize that historical slash-and-burn effect will still be reflected in lowered soil organic matter stocks and a differing forest stand structure, with a higher prevalence of deciduous tree species.
2 Materials and methods
2.1 Study area
In this study, we sampled an area of protected old-growth forests Autiovaara that belongs to Patvinsuo National park in Lieksa and Ilomantsi municipalities, North Karelia, eastern Finland (63°7’N, 30°40´E). The study area covers about 300 ha. Yearly average temperature in the study region is +2 °C, with January mean of –12 °C and July mean of +15.8 °C, and the area belongs to the southern edge of middle boreal zone (Ahti et al. 1968). Yearly precipitation varies between 500 and 800 mm, of which about half falls as snow (Ilmatieteen laitos 1991). Elevation a.s.l. in the area ranges from 170 to 270 m. Soil parent material is of morainic origin. Myrtillus and Vaccinium forest types sensu Cajander (1949) dominate the area (Lehtonen 1997). Forests in Autiovaara are mixed conifer forests of Scots pine (Pinus sylvestris L.) and Norway Spruce (Picea abies (L.) H. Karst.), with locally abundant European white birch (Betula pendula Roth), pubescent birch (Betula pubescens Ehrh.) and European aspen (Populus tremula L.), as described by Lehtonen (1997).
The area has been anthropogenically affected for hundreds of years, including large-scale and intensive slash-and-burn cultivation (PKMMKA 1899). Historical fire return interval during slash-and-burn period is estimated at 37–59 years for the Autiovaara areas not subjected to the practice, and as short as 11 years in areas that have been used for slash-and-burn (Lehtonen et al. 1996). The burning interval in non-slash-and-burn areas varied spatially and was related to the topography and the occurrence of slash-and-burn areas nearby (Lehtonen et al. 1996). Based on dendrochronological assessment, there have been no fires in Autiovaara after year 1900 (Lehtonen et al. 1996). After the cessation of slash-and-burn in the area at the end of 1800s, the forests were left to develop naturally and no forestry was practiced there.
2.2 Sample plots
We chose three separate areas (A1, A2, A3) from Autiovaara area, based on historical land-use maps (PKMMKA 1899). Areas were selected in such a way that they included one slash-and-burn subarea and an adjacent control subarea that was outside of the slash-and-burn site (Fig. 1). Slash-and-burn and control forest area outlines in Autiovaara were digitized from a georeferenced historic map (PKMMKA 1899, Fig. 2), using ArcMap v. 10.1. The spatial accuracy of the features in this old map is assumed to be within a few tens of meters based on an expert opinion (Jouni Aarnio, pers. comm. in 2017). Within each of these subareas, a systematic sampling was performed.
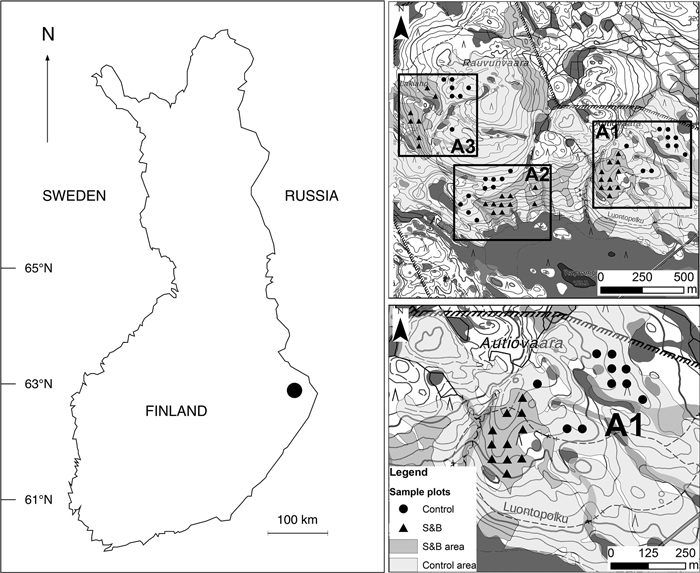
Fig. 1. Location of Autiovaara study area in Finland and the layout of sample plots in the terrain. S&B = slash-and-burn; A1, A2, A3 = separate areas in the terrain, where in each there was one slash-and-burn and one control subarea with several sample plots within. Contains data from the National Land Survey of Finland Basic Map Raster 06/2015.
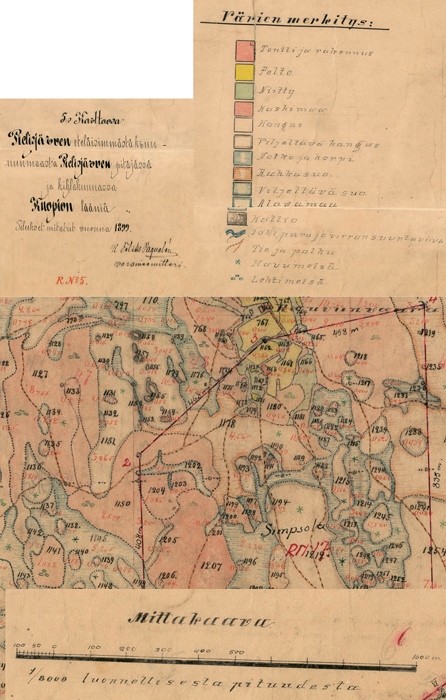
Fig. 2. Example of the old land use map. North-Karelian land survey bureau archive, Lieksa. Map unit 5. Pielisjärvi southernmost crownland, Reg. No. 29:5, PKMMKA 1899. Värien merkitys = legend: tontti ja rakennus = land parcel and building, pelto = field, niity = meadow, kaskimaa = slash-and-burn land, kangas = pine forest, viljeltävä kangas = upland forest suitable for agriculture, notko ja korpi = glen and spruce forest, rahkasuo = Sphagnum bog, viljeltävä suo = peatland suitable for agriculture, alavamaa = lowland, kallio = rock surface, joki, puro ja virran suuntaviiva = river, creek and the direction of the stream, tie ja polku = road and trail, havumetsä = coniferous forest, lehtimetsä = deciduous forest. Mittakaava = scale. View larger in new window/tab.
To select sample plots, a 50×50 m point grid was automatically produced from a starting point in one of the corners of the shapefile. Of all these points, the ones falling into the three slash-and-burn and three nearby control areas were selected. The points closer than 25 m to the edge of the forest polygon boundary or the hiking path were removed to address the possible inaccuracy of historical map material. Altogether we established 55 sample plots in three areas in each of the two classes: slash-and-burn and control (six subareas in total). Four out of these six subareas had 10–11 sample plots each, and two subareas – seven sample plots each.
2.3 Soil sampling
Soil was sampled in four subareas (two slash-and-burn and two control), in altogether 41 sample plots. Three out of these subareas had 10 sample plots each, and the one slash-and-burn subarea 11 sample plots. To characterize the main soil parameters in each sample plot, we took eight soil samples in the four cardinal directions (eight meters from the center), and four intercardinal directions (six meters from the center). In each of the soil sampling points, a soil core of the organic hemic and sapric, i.e. Oe/Oa horizon (humus layer) and uppermost 8–10 cm mineral soil was taken with a 5.7 cm diameter soil borer. Humus layer and mineral soil thickness were measured and then all the eight soil subsamples from a sampling plot were pooled and homogenized to two samples per sampling plot: humus layer and uppermost mineral soil. In cases when an obstacle (rock or roots) was encountered and it was impossible to obtain at least eight cm of uppermost mineral soil, we moved the soil sampling point closer to the center of the sample plot. Soil samples were stored in paper bags until they were transferred to laboratory.
2.4 Tree stand sampling
To characterize tree stand structure, we laid out a circular sample plot around the center point, 10 m in radius. Diameter at breast height (DBH) of all standing live trees taller than 1.3 m and >5 cm DBH was recorded, along with the species. Seedlings and saplings inside the sample plot were counted and their species identified. Non-lying dead wood was recorded as standing dead wood if taller than 1.3 m and as stumps if shorter. All stumps were included and measured as long as they were visible above ground layer and moss. For standing dead wood, DBH, and for stumps, the diameter at the top portion were measured. The height was measured for both. For lying dead wood, the length of the piece falling inside the sampling circle was recorded, as well as diameter at both ends. For all dead wood, species (when possible) were identified. Tree volumes (m3 ha–1) were calculated for canopy trees and dead wood groups.
The International Plant Names Index (International Plant Names Index 2004) was used for referring to tree and shrub species.
2.5 Laboratory analyses
Fresh (undried) soil samples were analyzed for pH in a water solution with a standard electrode. To prepare for soil organic matter (SOM) analysis, soil samples were oven-dried at 60 °C until constant weight, then sieved through 2-mm sieve. Resulting fine matter was analyzed for SOM by loss on ignition (LOI) at 550 °C for 2 hours. The preparation and LOI analysis of soil samples followed the standard method (Solid biofuels – Determination of ash content, EN 14775:2009) that was modified to suit the samples and conditions at hand (European Committee for Standardization 2009).
2.6 Data analysis
To test the slash-and-burn treatment effects on soil properties – humus layer thickness, SOM, pH and bulk density – we used linear mixed-effects model with a categorical predictor (slash-and-burn vs. control) and random groups defined by areas in the terrain to which the sampled forest plots belong (2 groups). Volumes of living trees by species and dead wood by type were compared between the treatments similarly as soil parameters, but three areas were used as random groups. Model residuals were checked for variance stability and normality visually by plotting. When residual normality was not met, the non-parametric unpaired Wilcoxon Signed-rank test, equivalent to Mann-Whitney U-test (Hollander and Wolfe 1973), was performed instead of the mixed-effects model. When the response variance was unequal between groups (slash-and-burn and control), the unequal variance Welch t-test without grouping (Ruxton 2006) was used instead of the mixed-effects model.
The effect of slash-and-burn disturbance on seedling count by species was assessed with a quasi-Poisson model with a categorical predictor (slash-and-burn vs. control) as the data showed overdispersion and with a zero-inflated negative binomial model for data with excess zeroes.
To compare the stands of living trees between slash-and-burn and control, we first described the stand structure by fitting a two-parameter Weibull distribution to the empirical DBH data in each sample plot using maximum likelihood estimator. We then extracted the shape and scale parameters of the fitted plot-wise Weibull distributions and ran a linear mixed-effects model (random-effect ANOVA) on the parameter values to reveal slash-and-burn effects. Fitting a two-parameter Weibull distribution to empirical tree stand DBH data is considered a robust means to explore tree stand structures and their differences (Sarkkola 2006). The shape parameter of Weibull distribution determines the shape of the distribution function. A larger shape parameter value gives a more left-skewed curve with a higher proportion of larger trees, while a smaller shape value gives a more right-skewed curve with a higher proportion of smaller trees. The scale parameter of Weibull distribution determines the scale and the peak of the distribution function. Larger values of the scale parameter define more “spread out” distribution functions typical to uneven-sized tree stand, while lower values – “narrower” distribution functions typical to even-sized stands.
Relationships between SOM stocks, tree stand volume and dead wood volume were evaluated with a linear mixed-effect model, including a power function for residual variance where required by residual structure. The random groups were the two adjacent sampling areas in the terrain.
Inferential data analysis and Weibull distribution estimations were performed in R (R Core Team 2013) and using “nlme” package (Pinheiro et al. 2017).
For the exploration of joint patterns and interrelationships within soil and tree stand structure parameters, we used principal components analysis (PCA) in Canoco 5 program (Šmilauer 2016) with replicates (i.e. sampling areas) and treatments independently added as environmental variables. We selected the linear PCA method because our set of variables measured represented different units.
3 Results
3.1 The impact of slash-and-burn on soil
Humus layer was 6 mm, or 14%, thinner in subareas of slash-and-burn disturbance than in controls (Fig. 3a) and it’s SOM stock was 1.1 kg m–2, or 25%, lower in slash-and-burn subareas (Fig. 3b). Mineral soil pH was lower in slash-and-burn subareas by 0.23 units, or 5%. (Fig. 3i). Other soil parameters did not show significant differences between the treatments (Fig. 3).
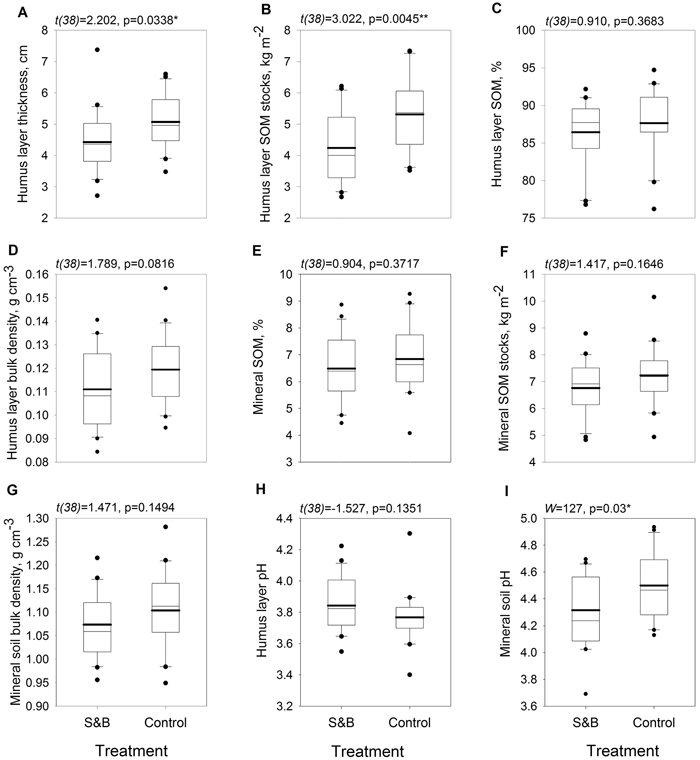
Fig. 3. Boxplots for soil parameters within slash-and-burn (S&B) and control areas with a thicker mean line: A = soil organic hemic and sapric, i.e. Oe/Oa (humus layer) thickness, cm; B = humus layer soil organic matter (SOM) stocks, kg m–2; C = humus layer SOM concentration, mass %; D = humus layer bulk density, g cm–3; E = uppermost 10 cm mineral SOM concentration, mass %; F = uppermost 10 cm mineral SOM stocks, kg m–2; G = uppermost 10 cm mineral soil bulk density, g cm–3; H = humus layer pH; I = uppermost 10 cm mineral soil pH. Test statistic and p-value for between-treatment differences given above the boxplot graphs. Significance levels: * = p < 0.05, ** = p < 0.005.
Several soil properties were correlated: PCA indicated a clear association between the overall soil characteristics and the treatment type (Fig. 4). Axis 1 was related mostly to the soil organic matter parameters and the treatments were quite different along this axis (Fig. 4), indicating that the main source of variation in the data was the treatment type. Variation in the soil parameters between the two sampling areas (A1, A2) on the other hand, was minimal (Fig. 4).
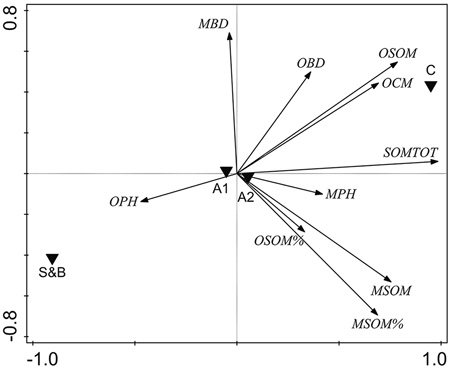
Fig. 4. Unconstrained PCA ordination of soil parameter data with two sampling areas and treatment types added as environmental variables. OPH = organic hemic and sapric, i.e. Oe/Oa (humus layer) pH; MBD = uppermost 10 cm mineral soil bulk density, g cm–3; OBD = humus layer bulk density, g cm–3; OSOM = humus layer soil organic matter stock, kg m–2; OCM = humus layer thickness, cm; SOMTOT = combined humus layer and uppermost 10 cm mineral SOM stock, kg m–2; MPH = uppermost 10 cm mineral soil pH; MSOM = uppermost 10 cm mineral SOM stock, kg m–2; MSOM% = uppermost 10 cm mineral SOM mass concentration percentage; OSOM% = humus layer SOM mass concentration percentage. S&B = slash-and-burn treatment; C = control treatment; A1, A2 = two sampling areas in the terrain, in each of which there was one slash-and-burn and one control sampled subarea.
3.2 Slash-and-burn effects on living tree and dead wood volume and regeneration
The volume of deciduous living trees was higher in slash-and-burn forests than in controls by 47.9 m3 ha–1, or 117% (Fig. 5, Fig. 6a). It was mainly comprised of birch, the volume of which was also higher by 48.2 m3 ha–1, or 158%, in slash-and-burn subareas than in controls (Fig. 5, Fig. 6b). Other standing tree species and groups (e.g. conifers, Scots pine, Norway spruce) did not have a significant volume difference between slash-and-burn and control sites (Fig. 5, Fig. 6), and varied mostly between the three sampling areas (A1, A2, A3) in Autiovaara (Fig. 5) in each of which there was one slash-and-burn and one control subarea sampled.
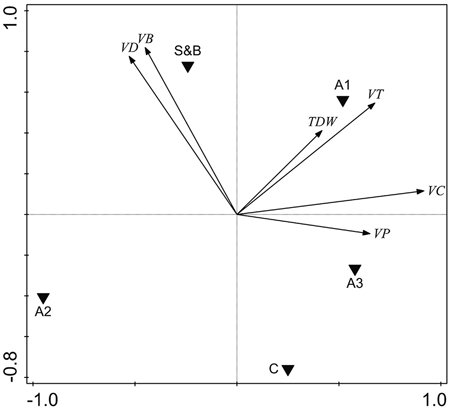
Fig. 5. Unconstrained PCA ordination of tree parameter data with three sampling areas and two treatment types added as environmental variables. VD = living deciduous tree volume, m3 ha–1; VB = living birch (Betula pendula and B. pubescens) volume, m3 ha–1; TDW = total dead wood volume, m3 ha–1; VT = total living tree volume, m3 ha–1, VC = living conifer tree volume, m3 ha–1; VP = living pine (Pinus sylvestris) volume, m3 ha–1. S&B = slash-and-burn treatment, C = control treatment; A1, A2, A3 = three sampling areas in the terrain, in each of which there was one slash-and-burn and one control sampled subarea.
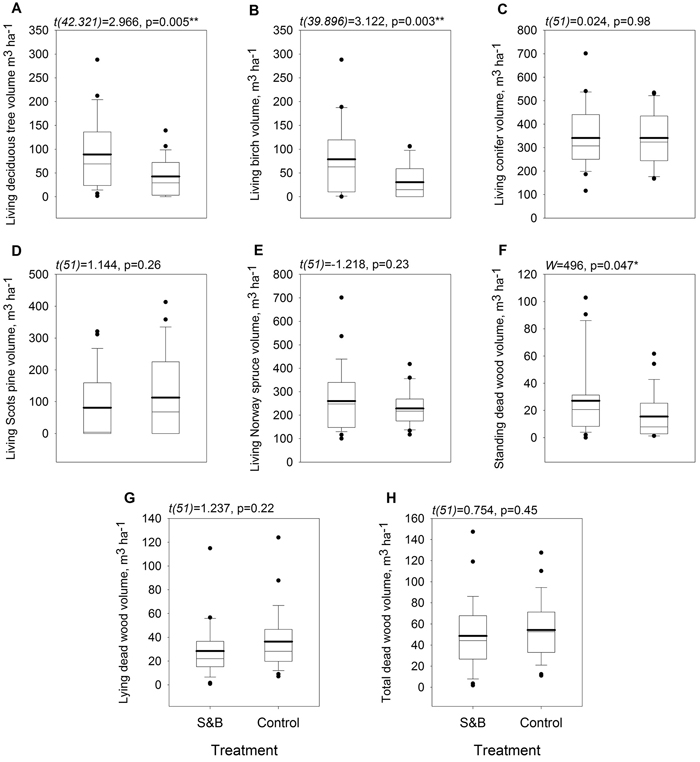
Fig. 6. Boxplots for tree stand parameters within slash-and-burn (S&B) and control areas with a thicker mean line: A: living deciduous tree volume, m3 ha–1; B: living birch (Betula pendula and B. pubescens) volume m3 ha–1; C: living coniferous tree volume, m3 ha–1; D: living Scots pine (Pinus sylvestris) volume, m3 ha–1; E: living Norway spruce (Picea abies) volume, m3 ha–1; F: standing dead wood volume, m3 ha–1; G: lying dead wood volume, m3 ha–1; H: total dead wood volume, m3 ha–1. Test statistic and p-value for between-treatment differences given above the boxplot graphs. Significance levels: * = p < 0.05, ** = p < 0.005.
Of all the dead wood categories, only standing dead wood volume was significantly higher by 11.6 m3 ha–1, or 75%, in slash-and-burn than in control areas (Fig. 6f).
None of tree seedling count models showed any significant difference between slash-and-burn and control sites (Supplementary file S1). Living tree and dead wood volume data are also summarized (Suppl. file S2).
3.3 Slash-and-burn effects on tree stand structure
The tree stands in slash-and-burn sites appeared to have slightly higher proportion of small trees (i.e. with the peak towards lower DBH) than the tree stands in control sites as shown with slightly more right-skewed DBH peak distribution (Fig. 7). However, the shape parameter of Weibull distributions, which indicates right- or left-skewness, fitted plot-wise to tree stand DBH only marginally differed between slash-and-burn and control areas (slash-and-burn mean = 0.668, control mean = 0.819, df = 51, t = 1.778, p = 0.08). The control sites appear to have more uneven-sized tree stand as seen in wider tree diameter distributions based on the mean Weibull scale parameter (slash-and-burn mean = 3.0688, control mean = 3.1787, df = 51, t = 2.393, p = 0.02).
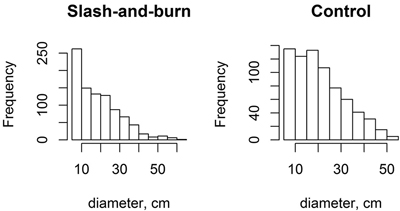
Fig. 7. Histograms for tree diameter at breast height (DBH) distributions (pooled data) for slash-and-burn and control sites.
3.4 Soil – living tree and soil – dead wood relationships
There was a significant negative relationship between humus layer thickness in cm (slope = –22.83, t(38) = –6.84, p < 0.0001), and humus layer SOM stock in kg m–2 (slope = –18.25, t(38) = –2.82, p = 0.0075) on one side, and birch standing live tree volume (m3 ha–1) on the other side (Fig. 8a, b). No significant relationship was found, however, between birch volume and mineral SOM stock.
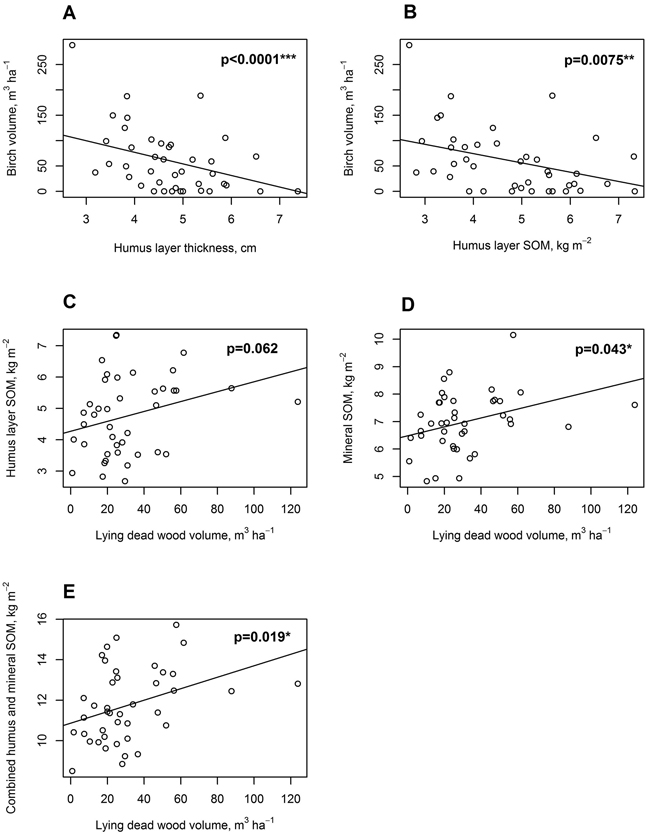
Fig. 8. Linear mixed-effects model regressions for soil, tree stand and dead wood parameters. A: Live standing birch volume, m3 ha–1, regressed on organic hemic and sapric, i.e. Oe/Oa horizon (humus layer) thickness, cm; B: Live standing birch volume, m3 ha–1, regressed on humus layer soil organic matter (SOM), kg m–2; C: Humus layer SOM, kg m–2 regressed on lying dead wood volume, m3 ha–1; D: Uppermost 10 cm mineral layer SOM, kg m–2, regressed on lying dead wood volume, m3 ha–1; E: Combined humus layer and uppermost 10 cm mineral layer SOM, kg m–2, regressed on lying dead wood volume, m3 ha–1.
There was an almost significant positive relationship between humus layer SOM stock in kg m–2 and lying dead wood volume in m3 ha–1 (slope = 0.016, t(38) = 1.93, p = 0.062, Fig. 8c). For mineral soil SOM stock, there was a positive significant relationship with lying dead wood volume (slope = 0.016, t(38) = 2.10, p = 0.043, Fig. 8d). Combined humus and mineral soil SOM stock had a positive significant relationship with lying dead wood volume as well (slope = 0.028, t(38) = 2.46, p = 0.019, Fig. 8e).
4 Discussion
In this study, we applied design that included control stands that were known to be never intentionally burned in immediate proximity to slash-and-burn stands to assess the impact of historically frequent anthropogenic burning disturbance on the boreal forests of Eastern Finland and their long-term recovery. While the proximity supports comparability due to biophysical conditions, it also poses a challenge related to slash-and-burn fires escaping and increasing fire frequency in nearby control areas as compared to common natural fire frequency for boreal Fennoscandia. In our control areas 37-to-59-year fire return interval has been estimated (Lehtonen et al. 1996), while a 112-year fire return interval was observed in the same region but with less human population (Haapanen and Siitonen 1978). Other estimates of boreal forest fire return interval from Sweden and Finland were also higher, in the interval of 90–128 years (Zackrisson 1977; Haapanen and Siitonen 1978; Engelmark 1984). More recent studies from boreal Fennoscandia show median fire return intervals at 79 years prior to year 1650, and 52 years after that (Niklasson and Granström 2000), i.e. comparable to observations in the control areas of this study. In pine forests of eastern Finnish Lapland fire cycle length, i.e. the time necessary for the burnt area to become equal to the area under study, was estimated at 350 years (Wallenius et al. 2010). In south-central Norway median fire return intervals were estimated at 73 years before 1600s and as low as 37 years during 1600–1700s, reflecting the peak of slash-and-burn cultivation during 17th century (Rolstad et al. 2017). Our control areas still feature a clearly longer fire return interval than slash-and-burn areas with fire return interval as short as 11 years, (Lehtonen et al. 1996) and thus a different disturbance regime.
Still after hundred years the forests studied here that have undergone historical slash-and-burn disturbance had a clearly lower humus layer thickness and humus layer SOM stock than forests in control areas. In the short term, fire disturbance in general is known to consume soil humus layer and thus reduce SOM stocks (Certini 2005; Neary et al. 2005). However, Johnson and Curtis (2001) found in their meta-analysis that more than 10 years after fire, SOM stocks can overshoot pre-fire levels due to enhanced post-disturbance litter input from rapid vegetation cover renewal. This, however, was not observed in our study. Our study features a much longer time since disturbance than ten years (i.e. ~100 years of recovery) and a preceding historical intensive disturbance regime of frequently repeated fires. Possibly these reasons, along with stabilization of litter input in mature forests, explain the lowered SOM stocks in former slash-and-burn stands that were observed here. Mineral soil SOM stocks did not differ significantly between slash-and-burn and control but were still somewhat lower for slash-and-burn (Fig. 3e, f), probably because mineral soil is less affected by fire due to the insulating effect of the humus layer (Neary et al. 2005).
Along with lower humus SOM stocks, birch standing tree volume (Fig. 6b) was significantly higher in slash-and-burn stands. As known for boreal Fennoscandian conditions, spruce is very sensitive to fire and is often replaced by broadleaved trees, such as silver and pubescent birch, after fire disturbance. Broadleaved species have lighter seeds that are more easily dispersed by wind than spruce seeds (Pennanen and Kuuluvainen 2002). This basic knowledge of Fennoscandian tree autecology and post-fire succession is confirmed by comparing mechanistic simulations of forest landscape structure with field measurements (Pennanen 2002; Pennanen and Kuuluvainen 2002). There is also similar evidence from boreal forests of Alaska that with increasing fire disturbance, tree stand species composition shifts from conifer-dominated to comprising more early-successional deciduous trees, mainly birch and aspen (Johnstone et al. 2010). In Alaska, this has been connected to the consumption of soil humus layer by fire that alters seedbed conditions (Johnstone et al. 2010). As fire activity is considered to increase in some regions of circumboreal forests (Westerling et al. 2006; Flannigan et al. 2009) due to changing climate (IPCC 2014), in such areas a landscape-level regime shift is predicted from conifer-dominated forests to forest that contain more deciduous trees (Johnstone et al. 2010). Fennoscandian boreal forests, however, are not predicted to face the increase in fire activity as in some other boreal areas (Girardin et al. 2009). Nonetheless, our results show that birch admixture that has formed in historical slash-and-burn areas was retained as large trees making up a significant portion of the stands a century later, i.e. the legacy has persisted at least within one birch cohort lifetime.
We observed a significantly higher volume of standing dead wood (i.e. snags) in slash-and-burn than in control areas. This could possibly reflect the higher propensity of birch to produce standing dead wood. It has been documented that birch usually recruits after a fire disturbance in mesic spruce-dominated sites in Finland (Sirén 1955). After about 100 years since last large-scale fire-disturbance, early-succession pioneer birch trees may have reached the age and stage where they produce large amounts of dead wood. Based on size structure and lack of birch seedlings, it may be that this enhanced amount of dead birch volume is a transient phenomenon, possibly similar to what is happening to aspen in many spruce-dominated old growth areas (Kouki et al. 2004; Lankia et al. 2012; Edenius and Ericsson 2015).
In contrast to birch standing tree volume that was strongly negatively associated with humus layer thickness and SOM stocks (Fig. 8a, b), lying dead wood volume was associated positively with mineral and mineral plus humus layer SOM stocks (Fig. 8d, e). This could reflect the transfer of dissolved organic matter from decomposing dead wood downwards through the humus layer and accumulation of it in mineral soil layer. Alternatively, this association could be mediated through the living tree stand dynamics as controlled by mineral SOM stocks. Disentangling these mechanisms possibly warrants further studies.
The small difference found in tree stand structure between slash-and-burn and control areas, i.e., the relatively higher number of small trees in slash-and-burn areas than in control areas, suggests that slash-and-burn disturbance regime has had an effect on tree stand structure, even though a century of recovery had probably already masked the original larger differences that were present immediately after cessation of slash-and-burn. Nonetheless, tree DBH distributions in both slash-and-burn and control areas have a reverse-J shape (Fig. 7), which is a characteristic of natural or near-natural forests with continuous tree recruitment in the absence of fire (Kuuluvainen et al. 1998; Uotila et al. 2002). Also, birch was an important constituent in forest stands analyzed here, even in control treatment with lower volumes as compared to slash-and-burn stands – the proportion of Betula spp. out of total live tree volume was 5, 9 and 11% within the three control subareas. Similar admixture of birch in old-growth spruce-dominated forests was observed in southern Finland, with ~8% of stand volume comprised by birch trees (Siitonen et al. 2000).
Similarly to the current study, long-term legacies of human land-use have been observed in northern Swedish forests that were used for ca. 300 years at low intensity by Sami reindeer herders before being abandoned ca. 100 years ago and let to develop without human impacts. These forests are located within the matrix of old-growth boreal forest that is considered one of the most pristine regions in northern Scandinavia. In the forests changed by Sami land use, both aboveground (shift to more early-successional plant species) and belowground (increase in soil organic matter cycling and nutrient availability) legacies have been observed (Freschet et al. 2014). This shows the persistent impacts of past cultural land use in the boreal zone roughly after a century of passive recovery, supporting the results of current study.
In general, our results point to the partial recovery of the boreal forest ecosystem during the ca. hundred years after the cessation of slash-and-burn disturbance regime, while some differences in soil and in living and dead tree stands are still apparent. Long-lasting effects of anthropogenic land-use are observed in many areas and ecosystems. A study on the naturally afforested abandoned agricultural fields in hemiboreal zone similarly concluded that soil retains morphological properties of agricultural land for a period of up to hundred years (Nikodemus et al. 2012). It is also not yet clear what long-term effects active restoration approaches, such as burning and dead wood creation, have on forest soil characteristics (Similä and Junninen 2012), and these effects need more studies based on careful planning (Čugunovs et al. 2017).
A space-for-time substitution study from a forest restoration site in Denmark projected deadwood amount to recover to natural levels within 50–100 years (Mazziotta et al. 2016). In our study, total dead wood amount was similar between slash-and-burn and control treatments (49.1 and 53.3 m3 ha–1, respectively) but lower than typical reported values for near-natural old-growth forests in Finland (84 m3 ha–1, [Rouvinen et al. 2005]) and natural old-growth forests in boreal European Russia (coarse woody debris volume of 117 m3 ha–1, [Kuuluvainen et al. 1998]). In our case, slash-and-burn stands can be expected to be younger than the untouched control stands, as they originated after the cessation of burning disturbance regime ~hundred years ago. This suggests that dead wood amount has effectively recovered to near-natural conditions within these ~hundred years of succession in former slash-and-burn stands. The species composition and decay stage of deadwood is, however, most probably different in former slash-and-burn sites as compared to control areas, due to the mortality of post-fire birch cohort in former slash-and-burn areas. The difficulties in dead wood species identification have precluded a more detailed qualitative analysis of dead wood pools. Moreover, the generally lower amount of dead wood in the study area than elsewhere in near-natural and natural boreal forests of Finland and northeastern Europe again possibly reflects the issue of fire escape to control areas of our study and some deviation from natural conditions even of our control stands (Lehtonen et al. 1996). Alternatively, the lower dead wood amounts could be caused by some local specificities (e.g. geologic or topographic) in the landscape.
There is a possibility that the effects related to tree stand characteristics observed in our study reflect only the recovery since last burning disturbance in slash-and-burn and control areas, i.e. not the cumulative legacies of multiple historical burnings. The background data that was used in this study relating to fire histories in Autiovaara area is not spatially explicit enough to show accurately the time of last fire within our sample plots. Nonetheless, we captured clear differences in tree stand characteristics between areas that were known to be used for slash-and-burn and the control areas outside of such land use. Soil characteristics, on the other hand, are expected to more likely reflect the legacies of cumulative disturbance regime than just the time since last disturbance.
We conclude that natural old-growth properties of Fennoscandian boreal forest ecosystem need more than hundred years to recover after a prolonged period of unnaturally frequent burning disturbance. While tree parameters can recover relatively close to the more natural state under passive rewildening, soil parameters appear to retain disturbance legacies more permanently and thus probably need more time than a century to return to pre-disturbance state. It is also clear that most of boreal Fennoscandian forests have been to some extent affected by human culture in the past and also before the peak period of slash-and-burn cultivation. It is thus imperative to consider the historical range of variation of ecosystem properties and choose the desired reference conditions to be attained in future management and/or conservation (Swetnam et al. 1999). Our study suggests that mesic spruce stands that have been used in slash-and-burn cultivation could recover their pre-slash-and-burn properties in the long-term, i.e. more than one century, but that the time needed for full recovery varies between different ecosystem properties.
Acknowledgements
We thank Metsähallitus (Parks and Wildlife Finland) for providing a trainee position for Ida Sara-Aho during the field work, and for the permission to conduct research in Patvinsuo National Park. Mihails Čugunovs’ work was supported by University of Eastern Finland, Doctoral Programme in Forests and Bioresources. Laura Pekkola’s work was funded by Tranfor-M student exchange programme. We thank Dr. Jouni Aarnio (Metsähallitus, Parks and Wildlife Finland) for his help in interpreting historical land-use maps. We also thank two anonymous reviewers for constructive comments on the manuscript.
References
Bürgi M., Östlund L., Mladenoff D.J. (2017). Legacy effects of human land use: ecosystems as time-lagged systems. Ecosystems 20(1): 94–103. https://doi.org/10.1007/s10021-016-0051-6.
Cajander A.K. (1949). Forest types and their significance. Acta Forestalia Fennica 56(5): 1–71. https://doi.org/10.14214/aff.7396.
Certini G. (2005). Effects of fire on properties of forest soils: a review. Oecologia 143(1): 1–10. https://doi.org/10.1007/s00442-004-1788-8.
Čugunovs M., Tuittila E.-S., Mehtätalo L., Pekkola L., Sara-Aho I., Kouki J. (2017). Variability and patterns in forest soil and vegetation characteristics after prescribed burning in clear-cuts and restoration burnings. Silva Fennica 51(1) article 1718. https://doi.org/10.14214/sf.1718.
Edenius L., Ericsson G. (2015). Effects of ungulate browsing on recruitment of aspen and rowan: a demographic approach. Scandinavian Journal of Forest Research 30(4): 283–288. https://doi.org/10.1080/02827581.2014.999823.
Engelmark O. (1984). Forest fires in the Muddus National Park (northern Sweden) during the past 600 years. Canadian Journal of Botany 62(5): 893–898. https://doi.org/10.1139/b84-127.
European Committee for Standardization (2009). Solid biofuels – determination of ash content, EN 14775:2009. A standard method. https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:31903,19930&cs=19BF142A1249BB4166A8F577333355837. [Cited 8 Dec 2016].
Flannigan M., Stocks B., Turetsky M., Wotton M. (2009). Impacts of climate change on fire activity and fire management in the circumboreal forest. Global Change Biology 15: 549–560. https://doi.org/10.1111/j.1365-2486.2008.01660.x.
Foster D., Swanson F., Aber J., Burke I., Brokaw N., Tilman D., Knapp A. (2003). The importance of land-use legacies to ecology and conservation. Bioscience 53(1): 77–88. https://doi.org/10.1641/0006-3568(2003)053%5B0077:TIOLUL%5D2.0.CO;2.
Freschet G.T., Östlund L., Kichenin E., Wardle D.A. (2014). Aboveground and belowground legacies of native Sami land use on boreal forest in northern Sweden 100 years after abandonment. Ecology 95(4): 963–977. https://doi.org/10.1890/13-0824.1.
Girardin M.P., Ali A.A., Carcaillet C., Mudelsee M., Drobyshev I., Hely C., Bergeron Y. (2009). Heterogeneous response of circumboreal wildfire risk to climate change since the early 1900s. Global Change Biology 15(11): 2751–2769. https://doi.org/10.1111/j.1365-2486.2009.01869.x.
Haapanen A., Siitonen P. (1978). Kulojen esiintyminen Ulvinsalon luonnonpuistossa. (Summary: Forest fires in Ulvinsalo strict nature reserve.) Silva Fennica 12(3): 187–200. https://doi.org/10.14214/sf.a14856.
Halme P., Allen K.A., Auniņš A., Bradshaw R.H.W., Brūmelis G., Čada V., Clear J.L., Eriksson A., Hannon G., Hyvärinen E., Ikauniece S., Iršėnaitė R., Jonsson B.G., Junninen K., Kareksela S., Komonen A., Kotiaho J.S., Kouki J., Kuuluvainen T., Mazziotta A., Mönkkönen M., Nyholm K., Oldén A., Shorohova E., Strange N., Toivanen T., Vanha-Majamaa I., Wallenius T., Ylisirniö A., Zin E. (2013). Challenges of ecological restoration: lessons from forests in northern Europe. Biological Conservation 167: 248–256. https://doi.org/10.1016/j.biocon.2013.08.029.
Hollander M., Wolfe D.A. (1973). Nonparametric Statistical Methods. New York, John Wiley & Sons. p. 68–75.
Holling C.S. (1996). Engineering resilience versus ecological resilience. In: Engineering within ecological constraints. Schulze P.C. (ed.). National Academy Press, Washington D.C. p. 31–43. 214 p.
Heikinheimo O. (1915). Kaskiviljelyn vaikutus Suomen metsiin. [The impact of swidden cultivation on forests in Finland]. Acta Forestalia Fennica 4. [In Finnish].
Hekkala A.-M., Tarvainen O., Tolvanen A. (2014). Dynamics of understory vegetation after restoration of natural characteristics in the boreal forests in Finland. Forest Ecology and Management 330: 55–66. https://doi.org/10.1016/j.foreco.2014.07.001.
Ilmatieteen laitos (1991). Tilastoja Suomen ilmastosta 1961–1990 – climatological statistics in Finland 1961–1990. Finnish Meteorological Institute, Helsinki.
International Plant Names Index (2004). http://www.ipni.org/. [Cited 3 May 2017].
IPCC (2014). Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri R.K., Meyer L.A. (eds.)]. IPCC, Geneva, Switzerland. 151 p.
Johnson D.W., Curtis P.S. (2001). Effects of forest management on soil C and N storage: meta analysis. Forest ecology and management 140(2–3): 227–238. https://doi.org/10.1016/S0378-1127(00)00282-6.
Johnson S., Strengbom J., Kouki J. (2014). Low levels of tree retention do not mitigate the effects of clearcutting on ground vegetation dynamics. Forest Ecology and Management 330: 67–74. https://doi.org/10.1016/j.foreco.2014.06.031.
Johnstone J.F., Chapin F.S., Hollingsworth T.N., Mack M.C., Romanovsky V., Turetsky M. (2010). Fire, climate change, and forest resilience in interior Alaska. Canadian Journal of Forest Research 40(7): 1302–1312. https://doi.org/10.1139/X10-061.
Josefsson T., Hörnberg G., Östlund L. (2009). Long-term human impact and vegetation changes in a boreal forest reserve: implications for the use of protected areas as ecological references. Ecosystems 12(6): 1017–1036. https://doi.org/10.1007/s10021-009-9276-y.
Kouki J. (2016). Ecology and biodiversity of boreal forests. http://forest.uef.fi/jarikouki/project_fire.htm. [Cited 28 Nov 2016].
Kouki J., Löfman S., Martikainen P., Rouvinen S., Uotila A. (2001). Forest fragmentation in Fennoscandia: linking habitat requirements of wood-associated threatened species to landscape and habitat changes. Scandinavian Journal of Forest Research 16 (S3): 27–37. https://doi.org/10.1080/028275801300090564.
Kouki J., Arnold K., Martikainen P. (2004). Long-term persistence of aspen and its associated threatened species are endangered in old-growth conservation areas in Finland. Journal for Nature Conservation 12(1): 41–52. https://doi.org/10.1016/j.jnc.2003.08.002.
Kouki J., Hyvärinen E., Lappalainen H., Martikainen P., Similä M. (2012). Landscape context affects the success of habitat restoration: large-scale colonization patterns of saproxylic and fire-associated species in boreal forests. Diversity and Distributions 18: 348–355. https://doi.org/10.1111/j.1472-4642.2011.00839.x.
Kuuluvainen T., Syrjänen K., Kalliola R. (1998). Structure of a pristine Picea abies forest in northeastern Europe. Journal of Vegetation Science 9: 563–574. https://doi.org/10.2307/3237272.
Lankia H., Wallenius T., Várkonyi G., Kouki J., Snäll T. (2012). Forest fire history, aspen and goat willow in a Fennoscandian old-growth landscape: are current population structures a legacy of historical fires? Journal of Vegetation Science 23(6): 1159–1169. https://doi.org/10.1111/j.1654-1103.2012.01426.x.
Lehtonen H. (1997). Forest fire history in North Karelia: a dendroecological approach. Doctoral dissertation. University of Joensuu, Faculty of Forestry.
Lehtonen H., Huttunen P. (1997). History of forest fires in eastern Finland from the fifteenth century ad – the possible effects of slash-and-burn cultivation. The Holocene 7(2): 223–228. https://doi.org/10.1177/095968369700700210.
Lehtonen H., Huttunen P., Zettenberg P. (1996). Influence of man on forest fire frequency in North Karelia, Finland, as evidenced by fire scars in Scots Pines. Annales Botanici Fennici 33: 257–263.
Lovén L., Äänismää P. (2006). Kolin kaskiopas. [Koli slash-and-burn guide]. 74 p. [In Finnish]. http://urn.fi/URN:ISBN:978-951-40-2017-9.
Mazziotta A., Heilmann-Clausen J., Bruun H.H., Fritz Ö., Aude E., Tøttrup A.P. (2016). Restoring hydrology and old-growth structures in a former production forest: Modelling the long-term effects on biodiversity. Forest Ecology and Management 381: 125–133. https://doi.org/10.1016/j.foreco.2016.09.028.
Neary D.G., Ryan K.C., DeBano L.F. (eds.) (2005, revised 2008). Wildland fire in ecosystems: effects of fire on soils and water. General Technical Report RMRS-GTR-42, vol. 4. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Ogden, UT. 250 p.
Niklasson M., Granström A. (2000). Numbers and sizes of fires: long-term spatially explicit fire history in a Swedish boreal landscape. Ecology 81(6): 1484–1499. https://doi.org/10.1890/0012-9658(2000)081%5B1484:NASOFL%5D2.0.CO;2.
Nikodemus O., Kasparinskis R., Kukuls I. (2012). Influence of afforestation on soil genesis, morphology and properties in glacial till deposits. Archives of Agronomy and Soil Science 59(3): 449–465. https://doi.org/10.1080/03650340.2011.638290.
Oldén A., Komonen A., Tervonen K., Halme P. (2017). Grazing and abandonment determine different tree dynamics in wood-pastures. Ambio 46(2): 227–236. https://doi.org/10.1007/s13280-016-0821-6.
Pennanen J. (2002). Forest age distribution under mixed-severity fire regimes – a simulation-based analysis for middle boreal Fennoscandia. Silva Fennica 36(1): 213–231. https://doi.org/10.14214/sf.559.
Pennanen J., Kuuluvainen T. (2002). A spatial simulation approach to natural forest landscape dynamics in boreal Fennoscandia. Forest Ecology and Management 164(1–3): 157–175. https://doi.org/10.1016/S0378-1127(01)00608-9.
Pinheiro J., Bates D., DebRoy S., Sarkar D., R Core Team (2017). nlme: linear and nonlinear mixed effects models. R package version 3.1-131. https://CRAN.R-project.org/package=nlme.
PKMMKA (1899). Pohjois-Karjalan maanmittauskonttorin arkisto, Lieksa. Kartanosa 5. Pielis-järven eteläisemmästä kruununmaasta, Rek. nro. 29:5. [North-Karelian land survey bureau archive, Lieksa. Map unit 5. Pielisjärvi southernmost crownland, Reg. No. 29:5.] [In Finnish].
R Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Rolstad J., Blanck Y.L., Storaunet K.O. (2017). Fire history in a western Fennoscandian
boreal forest as influenced by human land use and climate. Ecological Monographs 87(2): 219–245. https://doi.org/10.1002/ecm.1244.
Rouvinen S., Rautiainen A., Kouki J. (2005). A relation between historical forest use and current dead woody material in a boreal protected old-growth forest in Finland. Silva Fennica 39(1): 21–36. https://doi.org/10.14214/sf.393.
Ruxton G.D. (2006). The unequal variance t-test is an underused alternative to Student’s t-test and the Mann-Whitney U test. Behavioral Ecology 17(4): 688–690. https://doi.org/10.1093/beheco/ark016.
Sarkkola S. (2006). Stand structural dynamics on pristine and managed boreal peatlands. Doctoral dissertation. Dissertationes Forestales. https://doi.org/10.14214/df.29.
Siitonen J., Martikainen P., Punttila P., Rauh J. (2000). Coarse woody debris and stand characteristics in mature managed and old-growth boreal mesic forests in southern Finland. Forest Ecology and Management 128(3): 211–225. https://doi.org/10.1016/S0378-1127(99)00148-6.
Similä M., Junninen K. (2012). Ecological restoration and management in boreal forests – best practices from Finland. Metsähallitus, Natural Heritage Services, Vantaa. 54 p.
Sirén G. (1955). The development of spruce forest on raw humus sites in northern Finland and its ecology. Acta Forestalia Fennica 62(4). https://doi.org/10.14214/aff.7453.
Šmilauer P. (2016). Canoco5 support site. http://www.canoco5.com/. [Cited 16 Feb 2017].
Swetnam T.W., Allen C.D., Betancourt J.L. (1999). Applied historical ecology: using the past to manage for the future. Ecological Applications 9(4): 1189–1206. https://doi.org/10.1890/1051-0761(1999)009%5B1189:AHEUTP%5D2.0.CO;2.
Tikkanen O.-P., Ruokolainen A., Heikkilä R. (2014). Recovery of boreal forest structures near abandoned villages in Western White Sea Karelia, Russia. Scandinavian Journal of Forest Research 29(2): 152–161. https://doi.org/10.1080/02827581.2014.881543.
Turner II B.L., Lambin E.F., Reenberg A. (2007). The emergence of land change science for global environmental change and sustainability. Proceedings of the National Academy of Sciences 104(52): 20666–20671. https://doi.org/10.1073/pnas.0704119104.
UN environment (2017). Aichi biodiversity targets. https://www.cbd.int/sp/targets/. [Cited 6 April 2017].
Uotila A., Kouki J., Kontkanen H., Pulkkinen P. (2002). Assessing the naturalness of boreal forests in eastern Fennoscandia. Forest Ecology and Management 161: 257–277. https://doi.org/10.1016/S0378-1127(01)00496-0.
Vanha-Majamaa I., Lilja S., Ryömä R., Kotiaho J.S., Laaka-Lindberg S., Lindberg H., Puttonen P., Tamminen P., Toivanen T., Kuuluvainen T. (2007). Rehabilitating boreal forest structure and species composition in Finland through logging, dead wood creation and fire: The EVO experiment. Forest Ecology and Management 250(1–2): 77–88. https://doi.org/10.1016/j.foreco.2007.03.012.
Wallenius T.H., Kauhanen H., Herva H., Pennanen J. (2010). Long fire cycle in northern
boreal Pinus forests in Finnish Lapland. Canadian Journal of Forest Research 40(10): 2027–2035. https://doi.org/10.1139/X10-144.
Westerling A.L., Hidalgo H.G., Cayan D.R., Swetnam T.W. (2006). Warming and earlier spring increase western U.S. forest wildfire activity. Science 313(5789): 940–943. https://doi.org/10.1126/science.1128834.
Zackrisson O. (1977). Influence of forest fire on the north Swedish boreal forest. Oikos 29(1): 22–32. https://doi.org/10.2307/3543289.
Total of 61 references.

