Effect of stem rot on wood basic density, carbon, and nitrogen content of living deciduous trees in hemiboreal forests
Liepiņš J., Jaunslaviete I., Liepiņš K., Jansone L., Matisons R., Lazdiņš A., Jansons Ā. (2023). Effect of stem rot on wood basic density, carbon, and nitrogen content of living deciduous trees in hemiboreal forests. Silva Fennica vol. 57 no. 3 article id 23040. https://doi.org/10.14214/sf.23040
Highlights
- Stem rot significantly reduces the basic density of wood and increases its nitrogen content in living deciduous trees, while the carbon content appears irresponsive
- The effect of the distance from the pith on the basic density and nitrogen content of wood varies, depending on presence of discoloration or decomposition in the wood.
Abstract
While numerous studies have focused on analyzing various aspects of the carbon (C) budget in forests, there appears to be a lack of comprehensive assessments specifically addressing the impact of stem rot on the C budget of broadleaf tree species, especially in old-growth forests where stem rot is prevalent. One of the main challenges in accurately quantifying C losses caused by stem rot is the lack of precise data on the basic density and C content of decayed wood, which are crucial for converting decayed wood volume into biomass and C stocks. Using linear mixed-effects models, we examine the variability of wood basic density, C content, and nitrogen (N) content. Discolored and decomposed wood was collected from the stems of 136 living deciduous trees common in hemiboreal forests in Latvia. Our research indicates a noticeable reduction in the wood basic density, coupled with an increase in the N content within the stem wood throughout the decomposition process in birch (Betula spp.), European aspen (Populus tremula L.), grey alder (Alnus incana (L.) Moench), and common alder (Alnus glutinosa (L.) Gaertn.). While aspen wood showed a decreasing trend in C content as decay progressed, a pairwise comparison test revealed no significant differences in C content between discolored and decomposed wood for the studied species, unlike the findings for basic density and N content. This study emphasizes the need to account for stem rot in old-growth forest carbon budgets, especially in broadleaf species, and calls for more research on stem rot-induced carbon losses.
Keywords
wood specific gravity;
birch;
climate change mitigation;
biomass estimation;
alder;
aspen;
wood decay
-
Liepiņš,
Latvian State Forest Research Institute “Silava,” Rigas Street 111, LV-2169 Salaspils, Latvia
 https://orcid.org/0000-0003-3030-1122
E-mail
janis.liepins@silava.lv
https://orcid.org/0000-0003-3030-1122
E-mail
janis.liepins@silava.lv

-
Jaunslaviete,
Latvian State Forest Research Institute “Silava,” Rigas Street 111, LV-2169 Salaspils, Latvia
 https://orcid.org/0009-0000-7322-2729
E-mail
ieva.jaunslaviete@silava.lv
https://orcid.org/0009-0000-7322-2729
E-mail
ieva.jaunslaviete@silava.lv
-
Liepiņš,
Latvian State Forest Research Institute “Silava,” Rigas Street 111, LV-2169 Salaspils, Latvia
 https://orcid.org/0000-0002-1179-8586
E-mail
kaspars.liepins@silava.lv
https://orcid.org/0000-0002-1179-8586
E-mail
kaspars.liepins@silava.lv
-
Jansone,
Latvian State Forest Research Institute “Silava,” Rigas Street 111, LV-2169 Salaspils, Latvia
 https://orcid.org/0000-0003-2748-3797
E-mail
liga.jansone@silava.lv
https://orcid.org/0000-0003-2748-3797
E-mail
liga.jansone@silava.lv
- Matisons, Latvian State Forest Research Institute “Silava,” Rigas Street 111, LV-2169 Salaspils, Latvia E-mail roberts.matisons@silava.lv
-
Lazdiņš,
Latvian State Forest Research Institute “Silava,” Rigas Street 111, LV-2169 Salaspils, Latvia
 https://orcid.org/0000-0002-7169-2011
E-mail
andis.lazdins@silava.lv
https://orcid.org/0000-0002-7169-2011
E-mail
andis.lazdins@silava.lv
-
Jansons,
Latvian State Forest Research Institute “Silava,” Rigas Street 111, LV-2169 Salaspils, Latvia
 https://orcid.org/0000-0001-7981-4346
E-mail
aris.jansons@silava.lv
https://orcid.org/0000-0001-7981-4346
E-mail
aris.jansons@silava.lv
Received 2 August 2023 Accepted 25 September 2023 Published 28 September 2023
Views 62001
Available at https://doi.org/10.14214/sf.23040 | Download PDF

1 Introduction
The stem rot of living trees causes both economic and ecological losses to forests and forest ecosystems. It has detrimental effects on wood quality (Shortle and Dudzik 2012), tree vitality (Dobbertin 2005; Wei et al. 2022), and mechanical stability (Krisans et al. 2020), and can result in reduced tree growth and premature tree mortality (Roberts et al. 2020). Different factors can lead to stem rot, such as mechanical injuries (e.g. damage during thinning, pruning, skidding of wood), physical impacts (e.g. lightning strike, fire, hail, or ice), biological agents (e.g. pests, animals), and then fungi decompose the wood with the help of bacteria (Haq et al. 2022). Certain types of decay and discoloration seen in wood products can actually begin while the tree is still alive. There are two types of stem decays in standing timber: white rots and brown rots. White rots are more common, but brown rots are more worrisome because they cause a lot of damage to the wood strength (Zabel and Morrell 2020). Apart from research on the influence of stem rot on trees’ health status and the evaluation of urban tree safety, changes in the physicochemical properties of decayed wood and the release of carbon dioxide (CO2) during wood decomposition are also topical for various reasons. Fungi responsible for stem rot play a central role in the carbon and nitrogen cycling of forests, as they are the primary organisms capable of efficiently mineralizing all the cell wall components of wood into CO2 and water (Hietala et al. 2015).
The world’s forests serve as significant carbon sinks and play a critical role in mitigating global warming caused by the increasing concentration of atmospheric CO2 (Malhi et al. 2002; Pan et al. 2011). Reliable estimates of forest carbon pools are necessary to support political decisions and maintenance practices aimed to increase the ability of forests to absorb anthropogenic CO2 emissions. The primary source for carbon stock calculations in forests is national forest inventory (NFI) data. However, it has been recognized that the use of allometric equations – which typically do not account for the presence of internal decay – can lead to an overestimation of the carbon mass of individual trees and, consequently, the overall carbon estimates at larger scales for forests (Marra et al. 2018). Assumptions regarding the impact of carbon loss due to internal decay in living trees vary, and the available studies on this topic are limited. Matsuzaki et al. (2013) found internal decay had a non-significant effect on carbon stock in old coniferous stands in temperate rainforests in Canada, while a study of urban trees in Australia affirmed that the volume of decayed broadleaved trees could shrink up to 25%, which correspondingly led to a significant reduction of captured carbon (Orozco-Aguilar et al. 2018). In addition to these findings, a study in Poland found that the decay of 1 m3 of Norway spruce (Picea abies (L.) Karst.) wood would lead to the emission of approximately 106 kg of CO2 into the atmosphere (Sierota et al. 2018).
Stem rot can have implications not only for the carbon pool of living trees but also for the carbon pool of deadwood, which in turn affects the overall assessment of the carbon budget (Köster et al. 2015; Šenhofa et al. 2020). Additionally, the presence of internal decay can impact the fluxes of other greenhouse gases, including methane (CH4) and nitrogen oxides, primarily N2O (Barba et al. 2019). The importance of deadwood in carbon turnover within forest ecosystems has been recognized for a long time. This component is primarily quantified as part of NFI assessments (Russell et al. 2015). In response to the call for improvements in carbon accounting methodology, there has been extensive research conducted on the basic density of deadwood, as well as the release of CO2 through the decomposition of wood (Covey et al. 2016; Hietala et al. 2015; Köster et al. 2015; Neumann et al. 2023; Nilsson et al. 2002; Prescott et al. 2017; Stakėnas et al. 2020). In contrast, the study of internal decay in living trees has received considerably less attention in this context.
Old-growth forests play an invaluable role in biodiversity conservation and the provision of other ecosystem services (O’Brien et al. 2021). A core aim of the EU’s biodiversity strategy for 2030 is to increase the proportion of protected and strictly protected areas. The area of remaining primary and old-growth forests in the Europe is small, and to meet the strict protection area target, additional forest areas will be set aside to replenish the protected forest area network. Old-growth forests are usually considered a carbon sink (Luyssaert et al. 2008); however, natural disturbance regimes can have a strong effect on the longevity of these carbon reservoirs (Ķēniņa et al. 2023). Birches (Betula spp.), alders (grey alder (Alnus incana (L.) Moench), and common alder (Alnus glutinosa (L.)), and European aspen (Populus tremula L.) are so-called pioneer species characterized by a short lifespan (Rothkegel et al. 2020). These species cover more than half of the total forested area in Latvia (The Central Statistical Bureau 2023), and the proportion of overmatured birch, alder, and aspen stands will likely increase in the future to fulfill the goals of the EU’s biodiversity strategy for 2030. A deeper understanding of the chemical transformations that occur during wood decomposition within standing trees, along with an assessment of how sensitive tree carbon stock estimates are to the extent of internal decay in stems, will aid in evaluating the capacity of these stands to sustainably provide various ecosystem services, including carbon sequestration.
The objective of this study was to examine the variations in wood basic density, carbon (C), and nitrogen (N) concentrations within the discolored and decomposed wood present in the stems of living trees, focusing on broadleaved species commonly found in the hemiboreal region: birch, European aspen, grey alder, and common alder. We hypothesized that the mean values of basic density, C, and N concentrations would differ between discolored and decomposed wood. In addition to our objective, we sought to generate new knowledge that could contribute to the improvement of decay column biomass calculations: Specifically, to enhance the accuracy of assessing the reduction in carbon mass resulting from internal decay in living trees.
2 Materials and methods
2.1 Study sites and sampling design
The research was carried out in 29 randomly selected mature and old-growth forest stands of the four most dominant broadleaved species in Latvia: birch (downy birch (Betula pendula Roth) and silver birch (B. pubescens Ehrh.)), European aspen (Populus tremula L.), grey alder (Alnus incana (L.) Moench), and common alder (Alnus glutinosa (L.) Gaertn.) (Table 1). In Latvian forests, two tree species that resemble birch, namely silver birch and downy birch, are not individually identified in the forest inventory records. As a result, we have employed the inclusive term “birch or Betula” to refer to both. The selected stands were of natural origin, represented the typical growing conditions in Latvia, and located in forest sites belonging to the Forest research station that is why the selected sites are in groups (Fig. 1). According to data from the Latvian Environment, Geology, and Meteorology Centre, the annual average air temperature in Latvia is +5.9 °C. The year’s warmest month is July (17.0 °C) and the coldest is February (–4.6 °C). The climate is mild and humid, with four explicit seasons. The average annual precipitation in Latvia is 667 mm. European aspen and grey alder stands were classified as forests on fertile mineral soils, common alder stands were classified as forests on wet peat and drained peat soils, and Birch stands were classified as forests on both fertile mineral soils and drained peat soils.
| Table 1. Characteristics and sample sizes of studied tree species used to examine the variations in wood basic density, carbon (C), and nitrogen (N) concentrations within the discolored and decomposed wood present in the stems of living trees in Latvia. | |||||||
| Species | Number of trees (stands) | Age, years (range) | Diameter at breast height, cm (range) | Tree height, m (range) | Number of discs | Number of basic density samples | Number of C and N samples |
| Betula | 47 (8) | 85 (69–109) | 26.0 (9.5–44.7) | 24.1 (14.7–31.8) | 298 | 704 | 210 |
| Populus tremula | 19 (5) | 74 (69–89) | 37.7 (25.5–53.3) | 31.4 (28.7–33.5) | 276 | 801 | 211 |
| Alnus incana | 38 (9) | 49 (37–70) | 19.3 (14–28.5) | 20.8 (16.3–26) | 196 | 449 | 217 |
| Alnus glutinosa | 32 (7) | 90 (65–122) | 23.3 (13–40) | 23.3 (11.9–28.5) | 242 | 580 | 265 |
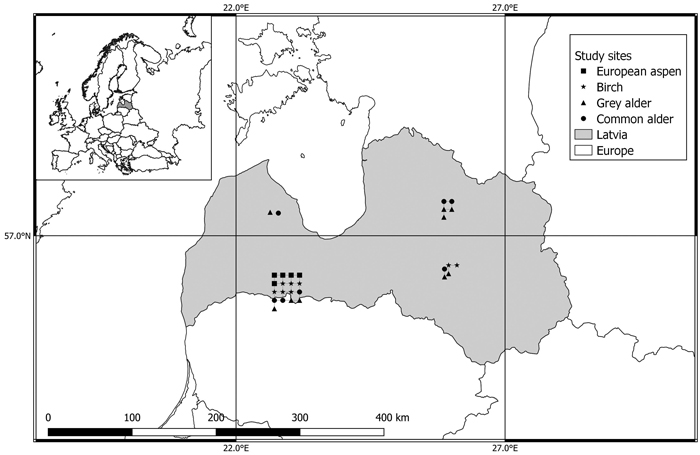
Fig. 1. Location of European aspen (Populus tremula), birch (Betula spp.), grey alder (Alnus incana), and common alder (Alnus glutinosa) study sites used to examine the variations in wood basic density, carbon, and nitrogen concentrations within the decayed wood present in the stems of living trees in Latvia.
For decay detection at the study sites, the stems of the dominant species in a 500-m2 sample plot were drilled horizontally at stump level by the Rinntech RESISTOGRAPH® R650 from three different directions towards the core. This is a reliable method of tree vitality assessment in which – by measuring the power consumption – it is possible to evaluate the change in wood density caused by internal decay (Allikmäe et al. 2017). A tree was classified as decayed if a decrease in density was detected in at least one of the drilling directions. The decayed trees were marked and felled for wood sampling. The stump height was defined as 1% of the measured tree height before felling. The field sampling was performed from April to August 2022.
2.2 Collection of decayed wood samples
After felling, the tree stems were cross-cut into 1-m logs starting from the base of the stem toward the tree top. The presence of internal decay was evaluated based on visual inspection of the log ends and also at a height of 1.3 m; if decay was judged as present, sample discs were collected.
In this study, according to the previously developed methodology (Arhipova et al. 2011, 2012), two conditions of wood were distinguished while collecting specimens from sampled discs for basic density, C, and N content analysis: (1) discolored wood without or with slightly changed mechanical properties (Fig. 2a) and (2) decomposed wood squeezable between two fingers (“spongy rot,” Fig. 2b). The detailed procedure and explanatory principles and schema for the extraction of wood specimens from cross-sectional discs were described in a study by Liepiņš et al. (2018). The width of the specimens from pith to bark was defined as 2 cm and wood specimens containing only discoloration or decomposed wood were prepared (Fig. 2c).

Fig. 2. Position of the specimens within the sample discs: (a) discolored wood; (b) decomposed wood; (c) wood specimens prepared for analyses of basic density.
2.3 Laboratory analyses
The density of wood specimens was determined using Precisa XB 220A scales bundled with a Precisa density determination set (part no: 350-8556). Before the density measurements were conducted, all wood specimens were immersed in water for 24 h to avoid absorption of water while taking measurements (Ilic et al. 2000). Each specimen was dried with soft paper and weighed in air and when submerged in water. The density of the immersed object was calculated according to Archimedes’ principle using these measurements. This method is particularly suitable for irregularly shaped objects. To calculate the basic density, the specimens were dried at 103–105 °C until a constant weight was achieved, which took around 4–5 days. Basic density helps provide a standardized measure of wood density, enabling comparisons between different wood samples and species.
At least 100 randomly selected specimens for each tree species and discolored or decomposed wood were cut into small pieces and ground into a homogenous powder. The wooden powder was analyzed for C and N content using the elemental analyzer (Elementar vario EL Cube, Elementar Analysensysteme GmbH, Germany), with a sample weight of approximately 30 mg. An elemental analyzer measures the amount of released CO2 and N2 through gas chromatography. The final values are expressed as the percentage of C or N in the dry wood powder.
2.4 Statistical analysis
The effect of stem rot (discolored or decomposed wood) and position of the specimen on wood basic density, C, and N content was determined using linear mixed-effects models (MANCOVA). The distance from the pith (numeric covariate), wood condition (factorial with two levels), and their interaction were used as the fixed effects. Due to hierarchical structure, trees were sampled in 5 to 9 stands for each tree species. In addition, because the data were unbalanced – and to avoid the issue of pseudoreplication (Arnqvist 2020) – trees and site were used as nested random effects, presuming both random slopes and random intercepts for the tested fixed effects.
The effect of wood decomposition (displayed as changes in basic density) on C and N content was determined using a separate linear mixed-effects model in a similar manner. The models were fit using a residual maximum likelihood technique and p-values for fixed effects derived with Satterthwaite approximations in a Type III analysis of variance. The R packages “lme4” and “lmerTest” were used for model analysis. The compliance of the models with statistical assumptions was checked by diagnostic plots. Predictor effect plots, implemented in the “effects” package in R, were used to provide a graphical summary for the fitted regression models.
Estimated marginal means (emmeans) and pairwise comparisons for each dependent variable were made using the R package “emmeans.” The level of statistical significance was set to 0.05. Conditional R2 (variance explained by the full model) and marginal R2 (variance explained by fixed effects) were calculated using the R package “MuMIn.” All statistical analyses were performed using R Studio software (R version 4.1.3 2022).
3 Results
The wood distance from the pith had a significant effect on the basic density of birch (p = 0.011), European aspen (p = 0.048), and grey alder (p = 0.035), while the stem rot significantly impacted (p < 0.05) both wood basic density and N content in all studied tree species (Table 2). In general, wood basic density tended to increase toward the bark, while the N content tended to decrease (Fig. 3). Both of these trends weakened or even disappeared as birch and common alder wood decomposed due to internal decay. The wood basic density of birch and the N content of common alder were both significantly influenced by the interaction between wood condition and distance from the pith. This indicated that the effect of the specimen’s position on wood basic density and N content varied depending on the presence of discoloration or decomposed wood. The decayed wood C content was not significantly impacted by the distance from pith in the affected area of internal decay for studied species. In the case of European aspen, the C content was significantly affected by the stem rot (p = 0.020), in contrast to the other tree species. In decomposed aspen wood, the carbon content tended to decrease.
| Table 2. Type III analysis of variance table with Satterthwaite’s method for the linear mixed-effects model to investigate the effect of wood condition and position of the specimen on wood basic density, carbon (C) and nitrogen (N) content. The table shows the number of degrees of freedom (DF), F-value, and p-value for each term in the model. Numbers in bold are statistically significant. | ||||||||||||
| Fixed effect variables | Betula | Populus tremula | Alnus incana | Alnus glutinosa | ||||||||
| DF | F-value | p-value | DF | F-value | p-value | DF | F-value | p-value | DF | F-value | p-value | |
| Basic density model | R2m(0.61), R2c(0.78) | R2m(0.68), R2c(0.75) | R2m(0.53), R2c(0.80) | R2m(0.37), R2c(0.57) | ||||||||
| Distance from pith | 6.33 | 12.8 | 0.011 | 3.17 | 9.9 | 0.048 | 11.02 | 5.8 | 0.035 | 192.24 | 1.0 | 0.308 |
| Wood condition | 8.78 | 75.1 | <0.001 | 2.18 | 123.4 | 0.006 | 10.82 | 87.9 | <0.001 | 6.40 | 31.8 | 0.001 |
| Distance:Wood condition | 114.59 | 8.0 | 0.006 | 289.59 | 0.6 | 0.425 | 375.90 | 3.4 | 0.067 | 210.65 | 0.1 | 0.725 |
| C content model | R2m(0.12), R2c(0.65) | R2m(0.15), R2c(0.26) | R2m(0.01), R2c(0.33) | R2m(0.37), R2c(0.57) | ||||||||
| Distance from pith | 4.04 | 3.0 | 0.159 | 0.73 | 7.1 | 0.295 | 3.97 | 0.4 | 0.562 | 1.46 | 0.3 | 0.653 |
| Wood condition | 5.90 | 5.4 | 0.059 | 22.15 | 6.3 | 0.020 | 5.60 | 0.1 | 0.808 | 7.23 | 0.2 | 0.641 |
| Distance:Wood condition | 166.43 | 1.3 | 0.260 | 12.29 | 0.3 | 0.570 | 114.65 | 0.3 | 0.595 | 134.17 | 3.2 | 0.076 |
| N content model | R2m(0.44), R2c(0.87) | R2m(0.55), R2c(0.64) | R2m(0.30), R2c(0.62) | R2m(0.28), R2c(0.58) | ||||||||
| Distance from pith | 3.62 | 5.9 | 0.079 | 2.39 | 2.1 | 0.269 | 6.20 | 4.0 | 0.092 | 3.79 | 3.3 | 0.147 |
| Wood condition | 5.51 | 24.8 | 0.003 | 21.08 | 49.7 | <0.001 | 10.33 | 16.0 | 0.002 | 6.38 | 6.5 | 0.041 |
| Distance:Wood condition | 174.27 | 0.1 | 0.817 | 76.12 | 0.1 | 0.714 | 151.29 | 0.2 | 0.693 | 159.18 | 6.0 | 0.015 |
| The interaction between the factors distance from pith and wood condition is represented as “Distance:Wood condition” | ||||||||||||
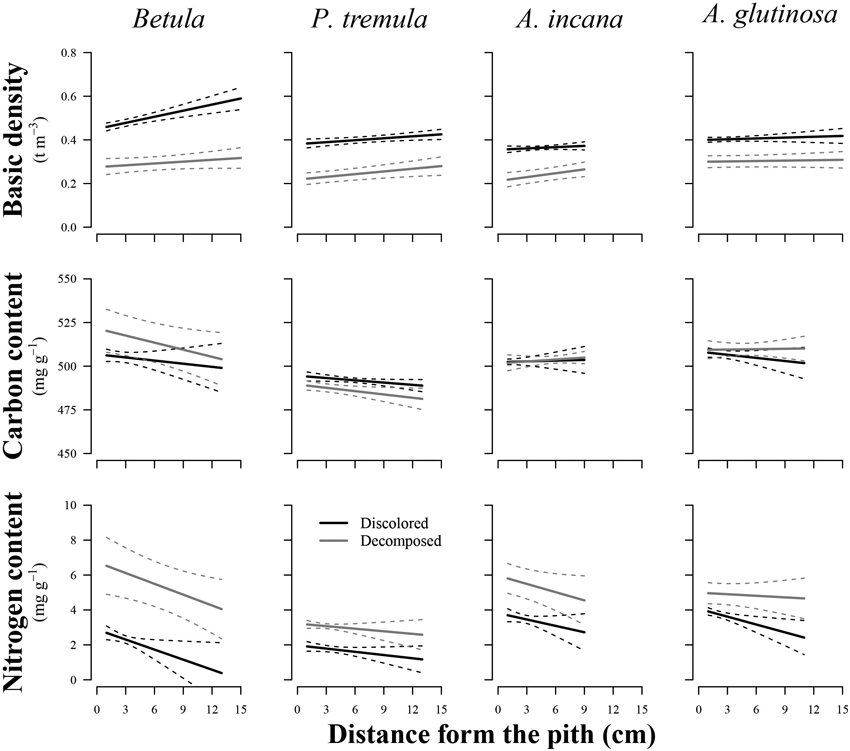
Fig. 3. Effect plots showing differences in basic density, carbon content and nitrogen content across discolored or decomposed wood and distance from pith for different tree species. The area between dotted lines indicates 95% confidence interval.
Marginal R2 and conditional R2 coefficients for some of our models suggested that – while the models fit the data reasonably well – substantial variation remained unexplained by the fixed-effect variables. The conditional R2 values for all the models were higher, indicating that a large portion of the variation in the dependent variable could be explained by the tree itself, saprotrophic or pathogenic fungi involved, and the specific stand (i.e., the location) in which the tree was growing.
Variations in basic density showed a significant effect (p < 0.05) on N content in all species examined (Fig. 4) (Table 3). The R2c values indicated a good relationship between the fitted and estimated values for studied species, explaining 45% to 86% of the variation in N content. In contrast, predicting C content was associated with higher prediction errors than N content, resulting in poorer R2c values (0.17 to 0.61) and R2m values (0.01 to 0.11). Basic density had a significant effect only on C content for European aspen (p = 0.015) and common alder (p = 0.025).
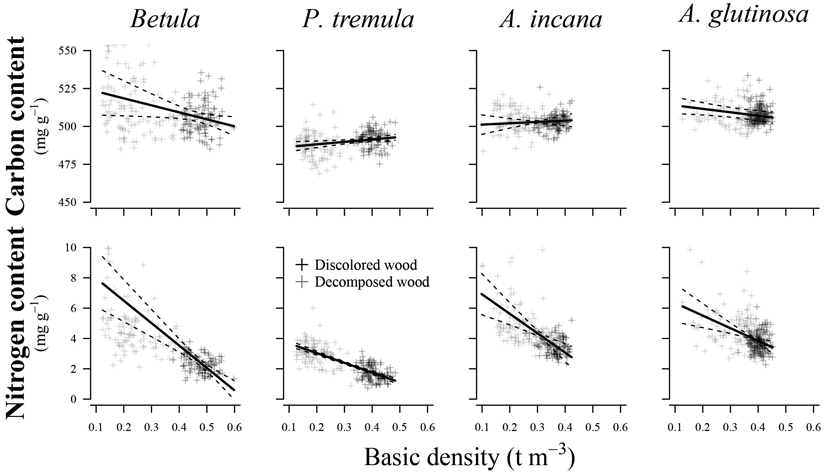
Fig. 4. Effect plots showing differences in carbon and nitrogen content across basic density for different tree species. The area between dotted lines indicates 95% confidence interval.
| Table 3. Type III analysis of variance table with Satterthwaite’s method for the linear mixed-effects model to investigate the effect of basic density on wood carbon (C) and nitrogen (N) content. The table shows the number of degrees of freedom (DF), F-value, and p-value for the model. Numbers in bold are statistically significant. | ||||||||||||
| Fixed effect variable | Betula | Populus tremula | Alnus incana | Alnus glutinosa | ||||||||
| DF | F-value | p-Value | DF | F-value | p-value | DF | F-value | p-value | DF | F-value | p-value | |
| C content model | R2m(0.11), R2c(0.61) | R2m(0.06), R2c(0.17) | R2m(0.01), R2c(0.32) | R2m(0.03), R2c(0.45) | ||||||||
| Basic density | 4.94 | 4.8 | 0.081 | 14.80 | 7.5 | 0.015 | 3.62 | 0.3 | 0.626 | 14.75 | 6.2 | 0.025 |
| N content model | R2m(0.53), R2c(0.86) | R2m(0.60), R2c(0.60) | R2m(0.37), R2c(0.56) | R2m(0.25), R2c(0.45) | ||||||||
| Basic density | 4.94 | 35.4 | 0.002 | 17.32 | 248.9 | <0.001 | 5.19 | 16.1 | 0.009 | 5.15 | 12.8 | 0.015 |
The results of the emmeans analysis showed that for basic density, all four species had a significant difference (p < 0.005) between groups (discolored and decomposed wood), with discolored wood having a higher mean density than decomposed wood (Table 4). Birch had the highest decrease in density as a result of decay (mean difference between groups = 0.196 g m–3), followed by European aspen (0.152 g m–3), grey alder (0.132 g m–3), and common alder (0.102 g m–3). The pairwise comparison test confirmed that there were no common trends among the examined species and no significant differences (p > 0.05) in the emmean values of C content between discoloration and decomposed wood. The highest emmean for C content was found in the decomposed birch wood (517.0 g kg–1), while the lowest was in the decomposed European aspen wood (486.4 g kg–1). In general, the C content in European aspen discolored and decomposed wood was lower compared to birch and both alder species. The emmean values of N content were significantly (p < 0.005) higher in decomposed wood for all studied species. Birch had the highest increase in N content as a result of decay (mean difference between groups = 3.8 g kg–3), followed by grey alder (2.0 g kg–3), European aspen (1.4 g kg–3), and common alder (1.3 g kg–3).
| Table 4. Analysis of estimated marginal means (emmean) and pairwise comparison for wood basic density, carbon content, and nitrogen content of examined species across discolored and decomposed wood. Numbers in bold are statistically significant. | ||||||
| Dependent variables | Species | Wood condition | emmean | Standard error | Pairwise comparison | |
| Mean difference | p-value | |||||
| Basic density, t m–3 | Betula | Discolored wood | 0.480 | 0.010 | 0.196 | <0.0001 |
| Decomposed wood | 0.284 | 0.017 | ||||
| Populus tremula | Discolored wood | 0.394 | 0.008 | 0.157 | 0.0029 | |
| Decomposed wood | 0.237 | 0.016 | ||||
| Alnus incana | Discolored wood | 0.360 | 0.005 | 0.132 | <0.0001 | |
| Decomposed wood | 0.228 | 0.016 | ||||
| Alnus glutinosa | Discolored wood | 0.403 | 0.006 | 0.102 | 0.0011 | |
| Decomposed wood | 0.301 | 0.014 | ||||
| Carbon content, mg g–1 | Betula | Discolored wood | 505.0 | 1.880 | –12.20 | 0.1062 |
| Decomposed wood | 517.0 | 5.860 | ||||
| Populus tremula | Discolored wood | 492.4 | 1.027 | 5.98 | 0.2439 | |
| Decomposed wood | 486.4 | 2.519 | ||||
| Alnus incana | Discolored wood | 503.0 | 1.430 | 0.02 | 0.9938 | |
| Decomposed wood | 503.0 | 1.700 | ||||
| Alnus glutinosa | Discolored wood | 507.0 | 1.350 | –2.90 | 0.2020 | |
| Decomposed wood | 510.0 | 2.090 | ||||
| Nitrogen content, mg g–1 | Betula | Discolored wood | 2.2 | 0.146 | –3.8 | 0.0044 |
| Decomposed wood | 6.0 | 0.728 | ||||
| Populus tremula | Discolored wood | 1.7 | 0.096 | –1.4 | 0.0174 | |
| Decomposed wood | 3.1 | 0.115 | ||||
| Alnus incana | Discolored wood | 3.4 | 0.187 | –2.0 | 0.0171 | |
| Decomposed wood | 5.5 | 0.470 | ||||
| Alnus glutinosa | Discolored wood | 3.6 | 0.137 | –1.3 | 0.015 | |
| Decomposed wood | 4.9 | 0.320 | ||||
4 Discussion
The decomposition of wood due to fungal metabolism is one of the key processes in forest ecosystems. It is responsible for nutrient cycling and the release of CO2 stored in woody biomass (Andlar et al. 2018; Rawlings et al. 2022). Biological degradation of lignocellulose during the decomposition or decay of wood leads to a decrease in basic wood density, as reported from studies on deadwood of the same deciduous species – birch, alders, and aspen – carried out in the Baltic region (Köster et al. 2015; Stakėnas et al. 2020). Our study also provides clear evidence of a reduction in wood density due to decomposition for all the studied species. At the same time, the observed basic density values of discolored wood were very similar to the average densities reported for intact wood in previous studies (Aosaar et al. 2011; Heräjärvi 2004; Heräjärvi and Junkkonen 2006; Liepiņš et al. 2017, 2023). This indicates that there are no significant alterations in wood properties at this initial stage of decay. This is in line with the general understanding that the discoloration of stem wood is caused by oxidation of the phenolic substances catalyzed by various enzymes produced by microbes (Hörnfeldt et al. 2010) and also induced by the plant as a defense reaction e.g. Smith (2015) that actually have minor effects on the mechanical properties of the wood (Duchesne et al. 2016). The basic density of decomposed wood was consistently lower for all studied species compared to discolored wood. Loss of wood density was higher for decayed birch and aspen wood (41% to 40%, respectively) than for grey alder and common alder (37% and 25%, respectively).
Our data revealed that the N content of decomposed wood was higher for all tree species, and there was a negative correlation between N content and wood basic density. The increase of N content in deadwood has been observed in previous studies (Holub et al. 2001; Köster et al. 2015). The recycling of wood by fungi, and import of N by rhizomorphs and mycelial cords of wood-decay fungi during early decomposition stages, contribute to the increase of N content in deadwood (Stenlid et al. 2008). Rinne et al. (2017) emphasized the role of N fixation and import from soil via wood-decay and mycorrhizal fungi as external flows in raising the N content of deadwood. While fungi are widely recognized as playing a dominant role in the decomposition of deadwood, it is important to note that several bacterial taxa also contribute to the accumulation of N in deadwood through N fixation (Vojtěch et al. 2021). Unlike the deadwood, the decay column within the trees has no direct contact with the soil, and the mechanisms responsible for nutrient cycling of decayed wood in living trees require additional exploration.
As previously reported, there is a negative correlation between wood density and C concentration (Martin et al. 2018). Our results on decay-affected wood showed a significant relationship between density and C content for two out of the four studied deciduous tree species (common alder and European aspen). The interconnection between basic density and C content was less pronounced than for N content, indicating that predicting the C content of decay-affected wood using basic wood density is associated with relatively high prediction error. Several factors may contribute to the notable amount of unexplained variation that affected the model’s performance. The decomposition of wood is a complex process involving various fungi, each with species-specific abilities to decompose lignin, cellulose, and hemicellulose (Fukasawa 2021). We know that wood chemical components have different C content; for example, higher amounts of lignin in the wood are associated with higher concentrations of C (Lamlom and Savidge 2003). Decay is traditionally categorized into white-rot, brown-rot, and soft-rot. For instance, brown rot decomposes cellulose and hemicellulose, while lignin remains relatively unchanged (Fukasawa 2021). Apparently, the stem rot can influence the C concentration in affected wood, and this issue should be addressed in future studies, particularly in the exploration of decayed wood in standing trees.
Wood basic density is also a highly variable trait. Among the variations observed between individual stems, sites, and between juvenile and mature wood (Saranpää 2003), it is important to consider both radial and axial variations within the stem (Liepiņš et al. 2017; Repola 2006). We found that the distance to the pith had a significant effect on the density of decay-affected wood for all species except common alder. The stem wood density variation in the area affected by rot is relatively low for common alder (Hakkila 1970; Liepiņš et al. 2023). This likely explains the absence of radial density variation in discolored wood in our study. The most prevalent decay fungi in living stems of common alder are I. radiatus and Armillaria sp., which caused significant heart rot in this tree species (Arhipova et al. 2012). Perhaps this can be attributed to the specificity of these fungi, which exhibit rapid and extensive wood decomposition. As a result, the distance from the pith does not appear to have an influence on the decomposed wood. Furthermore, distance from the pith had no effect on C or N content in the studied samples. This is an important insight for developing the methodology for C accounting in decayed stems. The absence of radial variation in C content in decay-affected wood simplifies the assessment of C loss in decayed standing trees, allowing for the use of weighted mean C content values for whole trees (Bārdule et al. 2021).
Reliable information on decay incidence within forest stands as well as methods for estimating the volumes of cavities and decay columns within tree stems are necessary for evaluating the impact of internal decay on carbon loss. So far, studies on the spread of discoloration and decay within tree stems or logs have primarily focused on assessing the economic impact and estimating the loss of timber quality caused by these issues (Hallaksela and Niemistö 1998; Schneider et al. 2008). Alnus, Betula, and Populus belong to diffuse-porous tree species associated with a relatively fast loss of mass during decomposition compared to ring-porous deciduous species and conifers (Edelmann et al. 2023). The incidence of decay within the stems of the former species can be very high and increasing with age (Worrall and Fairweather 2009). Decay was found in 69% of Populus tremuloides Michx. stems examined in Ontario, Canada (Basham 1958), while 91% of studied European aspen stems in Sweden were discolored (Johansson 2013). Research on the stem internal quality of alder in Latvia revealed 75.1% and up to 54% of decayed stems in common alder and grey alder forest stands, respectively (Arhipova et al. 2011, 2012). This highlights the need for further efforts to develop methods for carbon accounting in forests that take the decay of standing trees into consideration.
The tracking of the impact of heart rot on carbon stocks in living biomass and other carbon pools in forests remains a challenging task, especially when considering changing forest age structures. Heart rot, a type of decay that affects the inner core of trees, can significantly alter the carbon dynamics in forest ecosystems. However, its influence on carbon stocks is complex and not well understood. It has been observed that the concentration of CO2 in trees with heart rot was generally two times higher than in healthy trees (Hietala et al. 2015). This suggests that stem rot not only reduces the C stock of the tree but could also potentially increase the release of CO2 from the rotten wood into the atmosphere. One of the main challenges lies in accurately quantifying the extent of internal decay within individual trees and across forest stands. Heart rot is often hidden from external view, and its progression can vary widely, making it difficult to assess its impact on carbon stocks over time. Traditional forest inventory methods may not capture the subtle changes occurring within decaying trees, and this can lead to overestimation of forest carbon stock. To address this issue, more advanced technologies for detecting and monitoring heart rot in forests are needed. These could include various imaging techniques (e.g., tomography, ultrasound) or the use of remote sensing methods (e.g., LiDAR) to assess tree or stand conditions (Nicolotti et al. 2003; Soge et al. 2021). Models and simulations that account for changing forest dynamics – combined with data from decay monitoring techniques – can contribute to projecting the future impact of heart rot on carbon stocks, with implications for forest carbon management and climate change mitigation.
In summary, the results of this study emphasize the influence of stem rot on the structural and compositional properties of wood in living birch, aspen, grey alder, and common alder trees. Specifically, our findings reveal a distinct decrease in basic wood density (25–41%) and an increase in the N content (35–172%) of the stem wood during the decomposition process, depending on the tree species. The limited variation in carbon content within the stem wood does not provide sufficient support for the claim that the mean values of C content differ between discolored and decomposed wood, in contrast to the findings for basic density and N content. The decayed wood C and N concentration was not significantly impacted by the distance from pith in the affected area of internal decay for studied species. Overall, these findings contribute to improving the accuracy of biomass calculations for decayed trees, which is crucial for understanding C dynamics and forest ecosystem functioning.
Declaration of openness of research materials, data, and code
Data available on request from the corresponding author.
Author’s contributions
Conceptualization (J.L.; A.J.), data curation (I.J.; L.J.), data analysis (J.L; I.J.; R.M.), writing – original draft preparation (J.L.; I.J.; K.L.), visualization (L.J.; R.M.), writing – review and editing, (J.L.; AL), project administration (A.J.). All authors have read and agreed to the published version of the manuscript.
Acknowledgements
We would like to express our gratitude to the laboratory team for facilitating the analysis of wood specimens in the Latvian State Forest Research Institute “Silava” laboratory. This work was supported by Joint Stock Company “Latvia’s State Forests” research program “Carbon Turnover in Forest Lands”, grant number 5-5.5.1_001j_101_23_55.
Funding
This research was funded by European Regional Development Fund (ERDF) in accordance with contract no. 1.1.1.1/21/A/063 between Central Finance and Contracting Agency and Latvian State Forest Research Institute ‘Silava’, the project “Tool for assessment of carbon turnover and greenhouse gas fluxes in broadleaved tree stands with consideration of internal stem decay”.
References
Allikmäe E, Laarmann D, Korjus H (2017) Vitality assessment of visually healthy trees in Estonia. Forests 8, article id 223. https://doi.org/10.3390/f8070223.
Andlar M, Rezić T, Marđetko N, Kracher D, Ludwig R, Šantek B (2018) Lignocellulose degradation: an overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng Life Sci 18: 768–778. https://doi.org/10.1002/elsc.201800039.
Aosaar J, Varik M, Lõhmus K, Uri V (2011) Stemwood density in young grey alder (Alnus incana (L.) Moench) and hybrid alder (Alnus hybrida A. Br.) stands growing on abandoned agricultural land. Balt For 17: 89–94.
Arhipova N, Gaitnieks T, Donis J, Stenlid J, Vasaitis R (2011) Decay, yield loss and associated fungi in stands of grey alder (Alnus incana) in Latvia. Forestry 84: 337–348. https://doi.org/10.1093/forestry/cpr018.
Arhipova N, Gaitnieks T, Donis J, Stenlid J, Vasaitis R (2012) Heart-rot and associated fungi in Alnus glutinosa stands in Latvia. Scand J For Res27: 327–336. https://doi.org/10.1080/02827581.2012.670727.
Arnqvist G (2020) Mixed models offer no freedom from degrees of freedom. Trends Ecol Evol 35: 329–335. https://doi.org/10.1016/j.tree.2019.12.004.
Barba J, Bradford MA, Brewer PE, Bruhn D, Covey K, van Haren J, Megonigal JP, Mikkelsen TN, Pangala SR, Pihlatie M, Poulter B, Rivas-Ubach A, Schadt CW, Terazawa K, Warner DL, Zhang Z, Vargas R (2019) Methane emissions from tree stems: a new frontier in the global carbon cycle. New Phytol 222: 18–28. https://doi.org/10.1111/nph.15582.
Bārdule A, Liepiņš J, Liepiņš K, Stola J, Butlers A, Lazdiņš A (2021) Variation in carbon content among the major tree species in hemiboreal forests in Latvia. Forests 12, article id 1292. https://doi.org/10.3390/f12091292.
Basham JT (1958) Decay of trembling aspen. Can J Botany 36: 491–505. https://doi.org/10.1139/b58-045.
Covey KR, de Mesquita CPB, Oberle B, Maynard DS, Bettigole C, Crowther TW, Duguid MC, Steven B, Zanne AE, Lapin M, Ashton MS, Oliver CD, Lee X, Bradford MA (2016) Greenhouse trace gases in deadwood. Biogeochemistry 130: 215–226. https://doi.org/10.1007/s10533-016-0253-1.
Dobbertin M (2005) Tree growth as indicator of tree vitality and of tree reaction to environmental stress: a review. Eur J For Res 124: 319–333. https://doi.org/10.1007/s10342-005-0085-3.
Duchesne I, Vincent M, Wang XA, Ung C-H, Swift DE (2016) Wood mechanical properties and discoloured heartwood proportion in sugar maple and yellow birch grown in New Brunswick. Bioresources 11: 2007–2019. https://doi.org/10.15376/BIORES.11.1.2007-2019.
Edelmann P, Weisser WW, Ambarlı D, Bässler C, Buscot F, Hofrichter M, Hoppe B, Kellner H, Minnich C, Moll J, Persoh D, Seibold S, Seilwinder C, Schulze E-D, Wöllauer S, Borken W (2023) Regional variation in deadwood decay of 13 tree species: effects of climate, soil and forest structure. For Ecol Manage 541, article id 121094. https://doi.org/10.1016/j.foreco.2023.121094.
Fukasawa Y (2021) Ecological impacts of fungal wood decay types: a review of current knowledge and future research directions. Ecol Res 36: 910–931. https://doi.org/10.1111/1440-1703.12260.
Hakkila P (1970) Basic density, bark percentage and dry matter content of grey alder (Alnus incana). Commun Inst For Fenn 71.5: 1–33. http://urn.fi/URN:NBN:fi-metla-201207171103.
Hallaksela A, Niemistö P (1998) Stem discoloration of planted silver birch. Scand J For Res 13: 169–176. https://doi.org/10.1080/02827589809382973.
Haq IU, Hillmann B, Moran M, Willard S, Knights D, Fixen KR, Schilling JS (2022) Bacterial communities associated with wood rot fungi that use distinct decomposition mechanisms. ISME Communications 2, article id 26. https://doi.org/10.1038/s43705-022-00108-5.
Heräjärvi H (2004) Variation of basic density and Brinell hardness within mature Finnish Betula pendula and B. pubescens stems. Wood Fiber Sci 36: 216–227.
Heräjärvi H, Junkkonen R (2006) Wood density and growth rate of European and hybrid aspen in southern Finland. Balt For 12: 2–8.
Hietala AM, Dörsch P, Kvaalen H, Solheim H (2015) Carbon dioxide and methane formation in norway spruce stems infected by white-rot fungi. Forests 6: 3304–3325. https://doi.org/10.3390/f6093304.
Holub SM, Spears JDH, Lajtha K (2001) A reanalysis of nutrient dynamics in coniferous coarse woody debris. Can J For Res 31: 1894–1902. https://doi.org/10.1139/x01-125.
Hörnfeldt R, Drouin M, Woxblom L (2010) False heartwood in beech Fagus sylvatica, birch Betula pendula, B. papyrifera and ash Fraxinus excelsior – an overview. Ecol Bull 61–76.
Ilic J, Boland D, McDonald M, Downes G, Blakemore P (2000) Woody density: phase 1 - State of knowledge. NCAS technical report no.18.
Johansson T (2013) Discolored stems of 12–63-year-old European aspen (Populus tremula L.).
Ķēniņa L, Elferts D, Jaunslaviete I, Bāders E, Jansons Ā (2023) Sustaining carbon storage: lessons from hemiboreal old-growth coniferous and deciduous forest stands. Forest Sci 69: 158–166. https://doi.org/10.1093/forsci/fxac055.
Köster K, Metslaid M, Engelhart J, Köster E (2015) Dead wood basic density, and the concentration of carbon and nitrogen for main tree species in managed hemiboreal forests. For Ecol Manage 354: 35–42. https://doi.org/10.1016/j.foreco.2015.06.039.
Krisans O, Matisons R, Rust S, Burnevica N, Bruna L, Elferts D, Kalvane L, Jansons A (2020) Presence of root rot reduces stability of Norway spruce (Picea abies): results of static pulling tests in Latvia. Forests 11, article id 416. https://doi.org/10.3390/F11040416.
Lamlom SH, Savidge RA (2003) A reassessment of carbon content in wood: variation within and between 41 North American species. Biomass Bioenergy 25: 381–388. https://doi.org/10.1016/S0961-9534(03)00033-3.
Liepiņš J, Ivanovs J, Lazdiņš A, Jansons J, Liepiņš K (2017) Mapping of basic density within European aspen stems in Latvia. Silva Fenn 51, article id 7798. https://doi.org/10.14214/sf.7798.
Liepiņš J, Lazdiņš A, Liepiņš K (2018) Equations for estimating above- and belowground biomass of Norway spruce, Scots pine, birch spp. and European aspen in Latvia. Scand J For Res 33: 58–70. https://doi.org/10.1080/02827581.2017.1337923.
Liepiņš K, Liepiņš J, Ivanovs J, Bārdule A, Jansone L, Jansons Ā (2023) Variation in the basic density of the tree components of gray alder and common alder. Forests 14, article id 135. https://doi.org/10.3390/f14010135.
Luyssaert S, Schulze E-D, Börner A, Knohl A, Hessenmöller D, Law BE, Ciais P, Grace J (2008) Old-growth forests as global carbon sinks. Nature 455: 213–215. https://doi.org/10.1038/nature07276.
Malhi Y, Meir P, Brown S (2002) Forests, carbon and global climate. Philos Trans A Math Phys Eng Sci 360: 1567–1591. https://doi.org/10.1098/rsta.2002.1020.
Marra RE, Brazee NJ, Fraver S (2018) Estimating carbon loss due to internal decay in living trees using tomography: implications for forest carbon budgets. Environ Res Letters 13, article id 105004. https://doi.org/10.1088/1748-9326/aae2bf.
Martin AR, Doraisami M, Thomas SC (2018) Global patterns in wood carbon concentration across the world’s trees and forests. Nat Geosci 11: 915–920. https://doi.org/10.1038/s41561-018-0246-x.
Matsuzaki E, Sanborn P, Fredeen AL, Shaw CH, Hawkins C (2013) Carbon stocks in managed and unmanaged old-growth western redcedar and western hemlock stands of Canada’s inland temperate rainforests. For Ecol Manage 297: 108–119. https://doi.org/10.1016/j.foreco.2012.11.042.
Neumann M, Echeverria S, Hasenauer H (2023) A simple concept for estimating deadwood carbon in forests. Carbon Manag 14, article id 2197762. https://doi.org/10.1080/17583004.2023.2197762.
Nicolotti G, Socco LV, Martinis R, Godio A, Sambuelli L (2003) Application and comparison of three tomographic techniques for detection of decay in trees. J Arboric 29: 66–78. https://doi.org/10.48044/jauf.2003.009.
Nilsson SG, Niklasson M, Hedin J, Aronsson G, Gutowski JM, Linder P, Ljungberg H, Mikusiński G, Ranius T (2002) Densities of large living and dead trees in old-growth temperate and boreal forests. For Ecol Manage 161: 189–204. https://doi.org/10.1016/S0378-1127(01)00480-7.
O’Brien L, Schuck A, Fraccaroli C, Pötzelsberger E, Winkel G, Lindner M (2021) A review of scientific evidence to inform policy implementation. Final report. European Forest Institute. https://doi.org/10.36333/rs1.
Orozco-Aguilar L, Johnstone D, Livesley SJ, Brack C (2018) The overlooked carbon loss due to decayed wood in urban trees. Urban For Urban Green 29: 142–153. https://doi.org/10.1016/j.ufug.2017.09.008.
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala SW, McGuire AD, Piao S, Rautiainen A, Sitch S, Hayes D (2011) A large and persistent carbon sink in the world’s forests. Science 333: 988–993. https://doi.org/10.1126/science.1201609.
Prescott CE, Corrao K, Reid AM, Zukswert JM, Addo-Danso SD (2017) Changes in mass, carbon, nitrogen, and phosphorus in logs decomposing for 30 years in three rocky mountain coniferous forests. Can J of For Res 47: 1418–1423. https://doi.org/10.1139/cjfr-2017-0001.
Rawlings A, O’Connor E, Moody SC, Dudley E, Boddy L, Fowler MS, Fitzpatrick DA, Doyle S, Eastwood DC (2022) Metabolic responses of two pioneer wood decay fungi to diurnally cycling temperature. J Ecol 110: 68–79. https://doi.org/10.1111/1365-2745.13716.
Repola J (2006) Models for vertical wood density of Scots pine, Norway spruce and birch stems, and their application to determine average wood density. Silva Fenn 40: 673–685. https://doi.org/10.14214/sf.322.
Rinne KT, Rajala T, Peltoniemi K, Chen J, Smolander A, Mäkipää R (2017) Accumulation rates and sources of external nitrogen in decaying wood in a Norway spruce dominated forest. Funct Ecol 31: 530–541. https://doi.org/10.1111/1365-2435.12734.
Roberts M, Gilligan CA, Kleczkowski A, Hanley N, Whalley AE, Healey JR (2020) The effect of forest management options on forest resilience to pathogens. Front For Glob Change 3, article id 7. https://doi.org/10.3389/ffgc.2020.00007.
Rothkegel W, Ruppert O, Klemmt H-J, Tretter S (2020) Unterschätzte Pioniere. [Underestimated pioneers]. Bayerische Landesanstalt für Wald und Forstwirtschaft 53: 23–25.
Russell MB, Fraver S, Aakala T, Gove JH, Woodall CW, D’Amato AW, Ducey MJ (2015) Quantifying carbon stores and decomposition in dead wood: a review. For Ecol Manage 350: 107–128. https://doi.org/10.1016/j.foreco.2015.04.033.
Saranpää P (2003) Wood density and growth. In: Barnett JR, Jeronimidis G (eds) Wood quality and its biological basis. Blackwell Publishing & CRC Press, Oxford; Boca Raton, FL, pp 87–117.
Schneider R, Riopel M, Pothier D, Côté L (2008) Predicting decay and round-wood end use volume in trembling aspen (Populus tremuloides Michx.). Ann For Sci 65, article id 608. https://doi.org/10.1051/forest:2008042.
Šenhofa S, Jaunslaviete I, Šņepsts G, Jansons J, Liepa L, Jansons A (2020) Deadwood characteristics in mature and old-growth birch stands and their implications for carbon storage. Forests 11, article id 536. https://doi.org/10.3390/F11050536.
Shortle WC, Dudzik KR (2012) Wood decay in living and dead trees: a pictorial overview. Gen Tech Rep NRS-97. U.S. Department of Agriculture, Forest Service, Northern Research Station. https://doi.org/10.2737/NRS-GTR-97.
Sierota Z, Żółciak A, Małecka M, Sikora K, Damszel M (2018) An approach to calculate CO2 release through Norway spruce wood decay by Heterobasidion parviporum. Dendrobiology 79: 91–96. https://doi.org/10.12657/DENBIO.079.008.
Smith KT (2015) Compartmentalization, resource allocation, and wood quality. Curr For Rep 1: 8–15. https://doi.org/10.1007/s40725-014-0002-4.
Soge AO, Popoola OI, Adetoyinbo AA (2021) Detection of wood decay and cavities in living trees: a review. Can J For Res 51: 937–947. https://doi.org/10.1139/cjfr-2020-0340.
Stakėnas V, Varnagiryte-Kabasinskiene I, Sirgedaitė-Šėžienė V, Armolaitis K, Araminienė V, Muraškienė M, Zemaitis P (2020) Dead wood carbon density for the main tree species in the Lithuanian hemiboreal forest. Eur J For Res 139: 1045–1055. https://doi.org/10.1007/s10342-020-01306-3.
Stenlid J, Penttilä R, Dahlberg A (2008) Chapter 13: Wood-decay basidiomycetes in boreal forests: distribution and community development. In: Boddy L, Frankland JC, van West P (eds) British Mycological Society Symposia Series. Academic Press, pp 239–262.
The Central Statistical Bureau (2023) Forestry in 2020. https://stat.gov.lv/en/statistics-themes/business-sectors/forestry/publications-and-infographics/7269-forestry-2020. Accessed 20 September 2023.
Vojtěch T, Vendula B, Tomáš V, Mayuko J, Rubén L-M, Maria OML, Pedro SJ, Rainier HZ, Tomáš C, Ulisses N da R, Petr B (2021) Complementary roles of wood-inhabiting fungi and bacteria facilitate deadwood decomposition. mSystems 6, article id e01078-20. https://doi.org/10.1128/msystems.01078-20.
Wei Z, Halik Ü, Aishan T, Abliz A, Welp M (2022) Spatial distribution patterns of trunk internal decay of Euphrates poplar riparian forest along the Tarim River, northwest China. For Ecol Manage 522, article id 120434. https://doi.org/10.1016/j.foreco.2022.120434.
Worrall J, Fairweather M (2009) Decay and discoloration of aspen. FIDL 149.
Zabel RA, Morrell JJ (2020) Chapter twelve – decays originating in the stems of living trees. In: Zabel RA, Morrell JJ (eds) Wood microbiology, second edition). Academic Press, San Diego, pp 311–337. https://doi.org/10.1016/B978-0-12-819465-2.00012-7.
Total of 63 references.

