Genetic variation of resistance in Scots pine as possible solution against Diplodia sapinea
Terhonen E., Kujala S., Pyhäjärvi T., Sutela S. (2025). Genetic variation of resistance in Scots pine as possible solution against Diplodia sapinea. Silva Fennica vol. 59 no. 2 article id 25028. https://doi.org/10.14214/sf.25028
Highlights
- First evidence of genetic variation in Diplodia sapinea resistance among Scots pine
- Necrosis progression over time varied by maternal genotype, indicating heritable resistance traits
- Necrosis was significantly reduced when D. sapinea was predisposed at elevated temperature, but only at early infection stages.
Abstract
With ongoing climate change, the risk caused by both native, well-known pathogens and new, invasive ones is increasing. Diplodia sapinea (Fr.) Fuckel is responsible for Diplodia tip blight, a new fungal disease in Finland, that kills the current-year shoots of Scots pine (Pinus sylvestris L.). This can lead to the death of young trees and increase the susceptibility of trees of all ages to other stressors. Since D. sapinea spreads by airborne spores, it cannot be eradicated. In this study, we present the first screening to evaluate the potential for harnessing the genetic variation of Scots pine to improve its resilience against D. sapinea. Further, we wanted to test if predisposing this warm-preferring pathogen to higher temperature will increase its virulence. On the contrary, higher temperature initially reduced the virulence of D. sapinea, but the effect diminished over time. Based on necrosis length, we observed between-family variation in seedling resistance. These findings support the need for larger future trials to explore the potential for harnessing genetic variation to enhance resistance against D. sapinea.
Keywords
Pinus sylvestris;
climate change;
forest management;
Diplodia tip blight
-
Terhonen,
Natural Resources Institute Finland (Luke), Forest health and biodiversity, Latokartanonkaari 9, 00790 Helsinki, Finland
 https://orcid.org/0000-0002-9288-440X
E-mail
eeva.terhonen@luke.fi
https://orcid.org/0000-0002-9288-440X
E-mail
eeva.terhonen@luke.fi

-
Kujala,
Natural Resources Institute Finland (Luke), Forest tree breeding, Paavo Havaksen tie 3, 90570 Oulu, Finland
 https://orcid.org/0000-0003-0949-6156
E-mail
sonja.kujala@luke.fi
https://orcid.org/0000-0003-0949-6156
E-mail
sonja.kujala@luke.fi
-
Pyhäjärvi,
University of Helsinki, Faculty of Agriculture and Forestry, Department of Forest Sciences, Viikki Plant Science Centre, Latokartanonkaari 7, 00014 University of Helsinki, Finland
 https://orcid.org/0000-0001-6958-5172
E-mail
tanja.pyhajarvi@helsinki.fi
https://orcid.org/0000-0001-6958-5172
E-mail
tanja.pyhajarvi@helsinki.fi
- Sutela, Natural Resources Institute Finland (Luke), Forest health and biodiversity, Latokartanonkaari 9, 00790 Helsinki, Finland E-mail suvi.sutela@luke.fi
Received 17 June 2025 Accepted 21 October 2025 Published 5 November 2025
Views 9650
Available at https://doi.org/10.14214/sf.25028 | Download PDF

Supplementary Files
1 Introduction
The opportunistic pathogen Diplodia sapinea (Fr.) Fuckel has emerged as a sudden cause of disease outbreaks (Diplodia tip blight) in Scots pine (Pinus sylvestris L.) in Finland (Terhonen 2023), probably as an outcome of higher temperatures and lower precipitation during growing season (Terhonen et al. 2025). Asexual pycnidiospores (Brookhouser and Peterson 1970; Peterson 1977; Phillips et al. 2013) infect pine needles throughout the growing season (Brookhouser and Peterson 1970; Li et al. 2019). Also, elongating shoots are susceptible for D. sapinea infection before their lignification (Flowers et al. 2001; Oostlander et al. 2023). Infections may remain latent (Terhonen et al. 2021; Blumenstein et al. 2021a), or may develop to visible symptoms (Diplodia tip blight) if the host tree is under increased stress (e.g. drought) (Blumenstein et al. 2021b; Blumenstein et al. 2022). The optimal growth temperature of D. sapinea is between 25–30 °C (Bußkamp 2018), suggesting that higher temperatures may contribute to its increased virulence (Blumenstein et al. 2021b), leading to more disease symptoms. Generally, northern regions, such as Finland, are warming at a faster rate than the global average (Rantanen et al. 2022; Rantanen et al. 2025), creating new temperature records (Rantanen et al. 2025). As D. sapinea is a warm-preferring fungal pathogen (Bußkamp 2018; Terhonen et al. 2025), that can grow in temperatures as high as +40 °C (Milijašević 2006), it poses new risks to Scots pine under climate change. This suggests that, in the Nordic regions, Scots pines – previously considered less vulnerable to this pathogen due to the cooler climate – may face increasing disease pressure as conditions become more suitable for D. sapinea establishment and spread (Terhonen et al. 2025).
Scots pine is both ecologically and economically important tree species in Finland, making up approximately 50% of the total growing stock, with a volume of about 1250 million cubic meters (National Forest Inventory, 12/13rd). The current value (2024) of good quality Scots pine trunk is ~80 €/m³ (Luke, Statistic database: https://statdb.luke.fi/PxWeb/pxweb/en/LUKE/). The demand of Scots pine seedlings is increasing, and nurseries have started to increase the production amount (Finnish Food Authority 2023). This means 60 million seedlings annually and the number can be expected to increase. As a wind-pollinated species, Scots pine is characterized by high genetic variation within the populations, but also by genetically determined phenotypic differences along adaptive gradients (Pyhäjärvi et al. 2019). Scots pine growth also differs significantly among populations (provenances) under drought conditions (Taeger et al. 2013; Semerci et al. 2017). Pines’ chemical defenses consist of phenols, flavonoids, tannins, and volatile compounds such as the monoterpene α-pinene. The monoterpene composition of Scots pine is under strong genetic control (Yazdani et al. 1982; Baradat and Yazdani 1988), with the best-known chemotypes being: 1) high 3-carene & low α-pinene and 2) low 3-carene & high α-pinene (Bäck et al. 2012; Muona et al. 1986). Through their antimicrobial and antioxidant properties, these compounds can protect pines also from pathogens (Keeling and Bohlman 2006).
Diplodia tip blight is difficult to control. Currently there are no forest management practices available against D. sapinea in Finland. As it is spreading through asexual conidiospores produced in dead cones, needles and shoots, it is impossible to eradicate it from forests. The cumulative impact of D. sapinea on Scots pine health can result in economic losses. The disruption of tree crowns, and the death of shoots, seedlings, and eventually mature trees (Blumenstein et al. 2021a; Caballol et al. 2022; Brodde et al. 2023), lowers its market value. Genetic variation has crucial role in host-pathogen dynamics. Over generations, variation in resistance determines how populations of Scots pine can adapt to new pathogen. In the short term, it affects the rate of tree survival and growth at stand level and among regions. Finally, the information of genetic variation in resistance within natural and breeding populations may facilitate resistance breeding and choice of resistant seed sources.
The aim of this study is to assess the variation in resistance of Scots pine maternal lines to D. sapinea. We hypothesize that variation in susceptibility of Scots pine to D. sapinea, as measured here by necrosis length (cm) in shoots, has a genetic component. If significant differences are observed among maternal lines, this would support the hypothesis. While the current dataset is limited in size, the results will indicate the need and direction for further investigation. Our objective (1) is to compare necrosis length (cm) caused by D. sapinea among different Scots pine maternal lines in a common garden setting, to detect potential genetic variation in resistance. Another objective (2) is to test whether exposing D. sapinea to higher temperatures (35 °C versus 20 °C) increases the virulence of this warm-preferring pathogen.
2 Material and methods
2.1 Scots pine origin and inoculation experiment
The seed lots used here were collected from five different naturally regenerated populations and therefore represent the local natural variation (Table 1). Seed were sown in the beginning of growing season 2021 in a greenhouse at the Haapastensyrjä research station Natural Resources Institute Finland (Luke), Southern Finland. Seedlings were then grown outside the greenhouse in randomized block design for the first summer after which they were transferred to outdoor conditions in Viikki, Helsinki. During the second summer (2022) seedlings were planted in individual pots (volume 1 litre) and remained outside. In June 2023 (26.6.2023), after current-year growth had ceased, the seedlings were inoculated on the main shoot with D. sapinea (strain NCBI ID: OP103742) as described in Terhonen (2023). The fungus had been grown for 14 days at either +20°C or +35 °C before the inoculations. For each mother tree, three seedlings were inoculated with the +20 °C-grown fungus, three with the +35 °C-grown fungus, and three were treated with a mock control (agar plug without fungus). All seedlings from the same mother tree were half-siblings (pollinated by unknown fathers in their respective forests of origin). The seedlings were kept outdoor (outside greenhouse) and watered if needed to keep the soil moist. Developing necrosis in shoots was measured with a ruler to the nearest 0.1 cm, after two weeks and two months. Before inoculations, the height and growth of 2023 were measured (to nearest 0.1cm), and the number of side shoots were calculated. Fifteen seedlings were randomly chosen for re-isolation of D. sapinea conducted as described in Terhonen (2023).
| Table 1. Overview of used Scots pine seedlings: Mother ID used in this experiment as a number, location of mother (population) and Latitude. | ||
| Mother ID | Population | Latitude |
| 2 | Kolari, FI | 67.33° N |
| 5 | Kolari, FI | 67.33° N |
| 6 | Kolari, FI | 67.33° N |
| 8 | Kälviä, FI | 63.73° N |
| 9 | Kälviä, FI | 63.73° N |
| 10 | Kälviä, FI | 63.73° N |
| 14 | Punkaharju, FI | 61.75° N |
| 15 | Punkaharju, FI | 61.75° N |
| 19 | Loviisa, FI | 60.46° N |
| 20 | Loviisa, FI | 60.46° N |
| 22 | Loviisa, FI | 60.46° N |
| 24 | Loviisa, FI | 60.46° N |
| 26 | Norra Gullabo, SE | 56.45° N |
2.2 Statistical testing
All statistical analyses were performed using R version 4.2.2 (R Core Team 2023) (Supplementary file S1).
A linear model (LM) was used to analyse the effect of inoculation treatment and seedling traits on necrosis length (Y, cm). The model was fitted (Bates et al. 2015) as follows:
![]()
where: β0 = intercept, β1–4 = regression coefficient, Yi = necrosis length of seedling i (cm), Ti = inoculation treatment (fungi grown at +20 °C, +35 °C, or mock control (categorical)), Gi = total growth of the seedling during 2023 (cm), Si = number of side shoots, Mi = effect of Mother ID (categorical), and εi = residual error term.
Population was excluded from the model due to the low number of mother trees per population.
The necrosis data between mother IDs was assessed for each inoculation method (+20 °C and +35 °C), separately and combined, after 2 weeks and 2 months using the Shapiro–Wilk test. If the data were not normally distributed, the analysis was performed with the Kruskal–Wallis test, followed by post hoc pairwise comparison using the Dunn–Bonferroni method. For normally distributed data, an analysis of variance (ANOVA) was applied, followed by Tukey’s post hoc test.
3 Results
Fungal inoculation was considered unsuccessful if necrosis was absent (0 mm) after two weeks. As a result, five seedlings were discarded (Mother IDs: 26 × 2, 15, 20, and 22). Diplodia sapinea was reisolated from all selected seedlings. The growth of the year 2023, number of side shoots and length of the seedling did not affect to the necrosis length (Table 2). Necrosis caused by D. sapinea that was predisposed at elevated temperature was significantly lower after two weeks compared to the +20 °C treatment (Table 2, Fig. 1), but this difference was no longer significant after two months. Both fungal inoculation treatments resulted in significantly greater necrosis compared to the mock control (Table 2, Fig. 1).
| Table 2. Linear model estimates of factors affecting necrosis length (cm) in Scots pine seedlings two weeks and two months after pathogen, Diplodia sapinea, inoculation. Significance (Sig.) codes: *** p < 0.001, ** p < 0.01, * p < 0.05. | ||||||||||
| Necrosis after two weeks | Necrosis after two months | |||||||||
| Effect | Estimate | Std. Error | t value | Pr(>|t|) | Sig. | Estimate | Std. Error | t value | Pr(>|t|) | Sig. |
| (Intercept) | 1.8168464 | 0.3587807 | 5.064 | 1.81e–06 | *** | 1.77076 | 1.05986 | 1.671 | 0.097809 | |
| Treatment35 °C a | –0.5853122 | 0.1394796 | –4.196 | 5.76e–05 | *** | –0.50907 | 0.41203 | –1.236 | 0.219450 | |
| TreatmentControl a | –2.5058077 | 0.1404937 | –17.836 | < 2e–16 | *** | –4.10799 | 0.41503 | –9.898 | < 2e–16 | *** |
| MotherID5 b | 0.2806464 | 0.2909528 | 0.965 | 0.337016 | 0.99796 | 0.85950 | 1.161 | 0.248284 | ||
| MotherID6 b | 0.4971742 | 0.2854753 | 1.742 | 0.084570 | 0.40203 | 0.84331 | 0.477 | 0.634571 | ||
| MotherID8 b | 0.7866629 | 0.2734562 | 2.877 | 0.004885 | ** | 2.03923 | 0.80781 | 2.524 | 0.013116 | * |
| MotherID9 b | 0.4568134 | 0.2927768 | 1.560 | 0.121761 | 0.90711 | 0.86488 | 1.049 | 0.296715 | ||
| MotherID10 b | 0.2642211 | 0.2778694 | 0.951 | 0.343890 | 0.09965 | 0.82085 | 0.121 | 0.903615 | ||
| MotherID14 b | 0.2969386 | 0.3003453 | 0.989 | 0.325148 | –0.11514 | 0.88724 | –0.130 | 0.896999 | ||
| MotherID15 b | 1.1245038 | 0.2931321 | 3.836 | 0.000216 | *** | 3.09696 | 0.86593 | 3.576 | 0.000532 | *** |
| MotherID19 b | 0.4057178 | 0.3084283 | 1.315 | 0.191284 | 1.22802 | 0.91112 | 1.348 | 0.180676 | ||
| MotherID20 b | 0.7334483 | 0.3037714 | 2.414 | 0.017522 | * | 1.13470 | 0.89736 | 1.264 | 0.208909 | |
| MotherID22 b | 0.5289703 | 0.3162700 | 1.673 | 0.097455 | 1.10774 | 0.93428 | 1.186 | 0.238487 | ||
| MotherID24 b | 0.6928978 | 0.2886688 | 2.400 | 0.018176 | * | 1.79427 | 0.85275 | 2.104 | 0.037804 | * |
| MotherID26 b | –0.1079628 | 0.2975640 | –0.363 | 0.717481 | –0.06853 | 0.87902 | –0.078 | 0.938013 | ||
| Growth c | 0.0248811 | 0.0325944 | 0.763 | 0.446997 | 0.02789 | 0.09629 | 0.290 | 0.772640 | ||
| Length d | –0.0001307 | 0.0254071 | –0.005 | 0.995906 | 0.04395 | 0.07505 | 0.586 | 0.559486 | ||
| SideShoots e | –0.0068661 | 0.0349079 | –0.197 | 0.844456 | 0.03422 | 0.10312 | 0.332 | 0.740653 | ||
| a Difference between inoculation treatment compared to the reference D. sapinea in 20 °C b Difference in MotherID, the reference is the maternal line MotherID2 c Effect of seedling current year growth (cm) on lesion length (cm) d Effect of seedling length (cm) on lesion length (cm) e Effect of number of side shoot on lesion length (cm) | ||||||||||
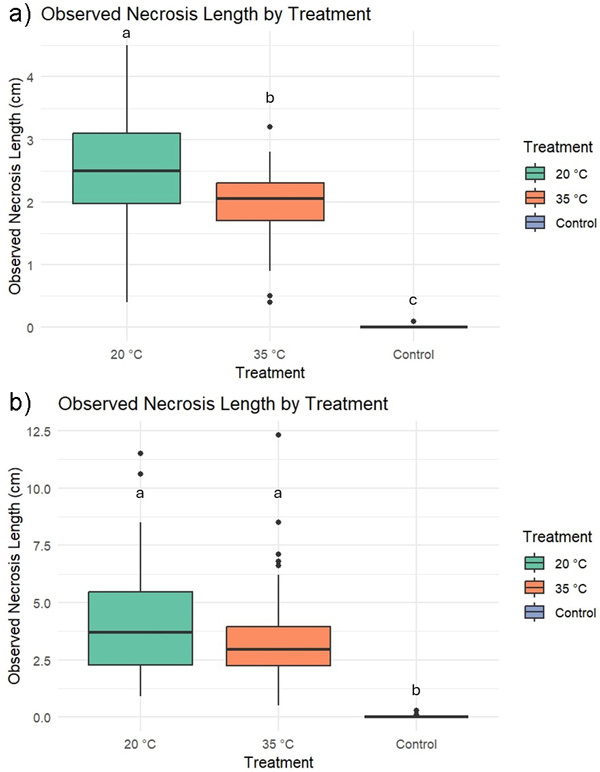
Fig. 1. Observed necrosis in three-year-old Scots pine seedlings subjected to different treatments: Diplodia sapinea grown at +20 °C, +35 °C, or mock control. Measurements were taken after two weeks (a) and two months (b).
The offsprings of Mother tree ID 15 was found to be most susceptible to D. sapinea (Table 2, Fig. 2). This was confirmed with ANOVA (+20 °C, 2 weeks), as the necrosis in Mother ID 15 was significantly higher than in Mother ID 2 (p = 0.017) and 26 (p = 0.007). Combined data (+20 °C and +35 °C, 2 weeks) showed that Mother ID 15 had meaningfully longer necrosis than Mother ID 2 (p = 0.004), 10 (p = 0.046) and 26 (p = 0.004).This was consistent with the combined necrosis data (+20 °C and +35 °C) after two months, where Mother ID 15 showed significantly higher necrosis than Mother IDs 2 (p = 0.0143), 10 (p = 0.0158) and 26 (p = 0.0461). Other comparisons between mother IDs did not show significant differences in necrosis (ANOVA or Kruskal–Wallis, depending on data distribution) at 20 °C after 2 months or at 35 °C after 2 weeks or 2 months. Moderate susceptibility was observed also in offsprings of Mother IDs 8 and 24 after two weeks and months (Table 2, Fig. 2). Seedlings from Mother ID 2 and 26 had the lowest necrosis, and the necrosis length did not increase significantly over time (Fig. 2).
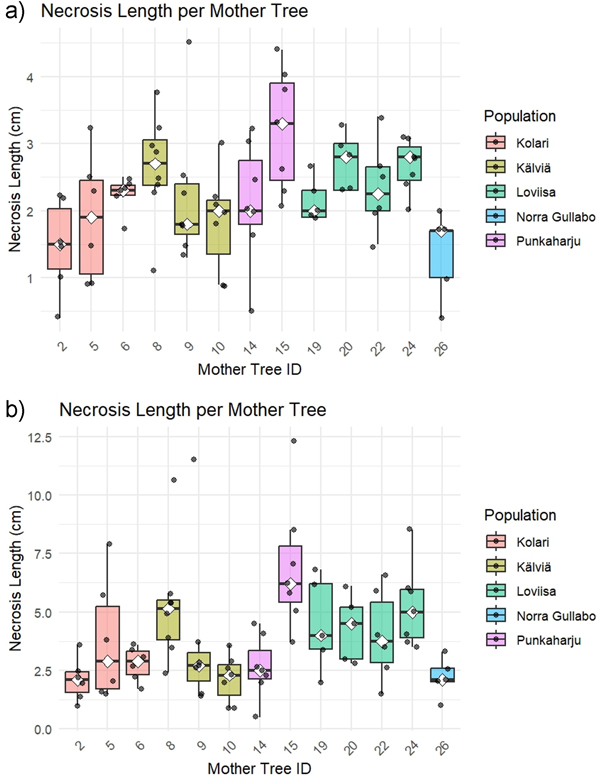
Fig. 2. Observed necrosis in three-year-old Scots pine seedlings from different mother trees. Measurements were taken after two weeks (a) and two months (b). To better illustrate the impact of Diplodia sapinea, necrosis lengths from both treatments were combined, and mock-control necrosis (0 cm) was excluded from the figure.
4 Discussion
Genetic variation in Scots pine can serve as a tool against D. sapinea. Previous studies have shown that Scots pine families exhibit significant differences in resistance to Dothistroma septosporum (Dorogin) M. Morelet, indicating that this trait is heritable (Perry et al. 2016). Scots pine populations from Central Europe show genetic variation both within and between populations in their susceptibility to pine twist rust (Melampsora pinitorqua Rostr.) (Quencez and Bastien 2001). In populations of Scots pine originating from Finland the difference in rust resistance was found between populations, but there was still variation among trees within each population (Andersson and Danell 1997). In Sweden, Scots pine blister rust (Cronartium pini (Willd.) Jørst) resistance was shown to have considerable narrow-sense heritability (Persson et al. 2024). Stein Åslund et al. (2025) observed in the field that certain Scots pine trees consistently exhibited lower Diplodia tip blight symptoms, suggesting the presence of genetic variation in tolerance that may enhance resilience. Due to the uneven number of maternal lines across our populations, we were unable to detect population-level differences. However, there was significant variation among half-sib families, indicating that genetic variation can have important role in host-pathogen interaction. As D. sapinea is a new pathogen in Finland (Terhonen 2023) and benefits from a warmer climate (Bußkamp 2018; Terhonen et al. 2025), genetic variation in resistance could be harnessed in Scots pine breeding for future conditions.
Diplodia sapinea has been shown to grow in temperature as high as +40 °C (Milijašević 2006), optimal growth being +30 °C (Bußkamp 2018). Therefore, we considered a +5 °C increase above the optimal growth temperature representing “warmer” conditions for D. sapinea in this experiment. The ability to cause necrosis decreased after exposed to warmer conditions, likely due to stress exceeding the pathogen’s optimal range. However, this effect was temporary, and the impact diminished over time, suggesting delayed pathogenicity after heat stress. The climatic parameters required for Finnish D. sapinea strains to (a) establish themselves and (b) become pathogenic are still unknown.
5 Conclusion
Significant variation among half-sib families suggests genetic variation related to resistance or susceptibility to D. sapinea in Scots pine. Larger data from both natural and breeding populations of Scots pine need to be studied to verify heritability of resistance against D. sapinea and to determine whether the variation describes host resistance or susceptibility.
Acknowledgements
We would like to thank Tuija Hytönen, Katri Leino, and Linda Mutanen for excellent technical assistance.
Authors’ contributions
Eeva Terhonen: Writing – original draft, methodology, formal analysis, data curation, conceptualization. Sonja Kujala: Writing – review & editing, methodology, data curation. Tanja Pyhäjärvi: Writing – review & editing, methodology, data curation. Suvi Sutela: Writing – review & editing, methodology, data curation.
Declaration of the use of generative artificial intelligence and AI-assisted technologies in the writing process
During the preparation of this work, the authors used OpenAI’s ChatGPT in order to improve the accuracy and clarity of the English language. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the published article.
Declaration of openness of research materials, data, and code
The data used in this article are openly available at https://doi.org/10.23729/fd-f7666f35-f2d9-3b9e-a0f9-68c06775879a.
Funding
This work was funded by Natural Resources Institute Finland (Luke) and Alfred Kordelin Foundation.
References
Andersson B, Danell Ö (1997) Is Pinus sylvestris resistance to pine twist rust associated with fitness costs or benefits? Evolution 51: 1808–1814. https://doi.org/10.1111/j.1558-5646.1997.tb05104.x.
Bäck J, Aalto J, Henriksson M, Hakola H, He Q, Boy M (2012) Chemodiversity of a Scots pine stand and implications for terpene air concentrations. Biogeosciences 9: 689–702. https://doi.org/10.5194/bg-9-689-2012.
Baradat Ph, Yazdani R (1988) Genetic expression for monoterpenes in clones of Pinus sylvestris grown on different sites. Scand J For Res 3: 25–36. https://doi.org/10.1080/02827588809382492.
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48. https://doi.org/10.18637/jss.v067.i01.
Blumenstein K, Bußkamp J, Langer G, Langer E, Terhonen E (2021a) The Diplodia tip blight pathogen Sphaeropsis sapinea is the most common fungus in Scots pines’ mycobiome irrespective of health status – a case study from Germany. J Fungi 7, article id 607. https://doi.org/10.3390/jof7080607.
Blumenstein K, Bußkamp J, Langer GJ, Schlößer R, Parra Rojas NM, Terhonen E (2021b) Sphaeropsis sapinea and associated endophytes in Scots pine: interactions and effect on the host under variable water content. Front For Glob Change 4, article id 655769. https://doi.org/10.3389/ffgc.2021.655769.
Blumenstein K, Bußkamp J, Langer G, Terhonen E (2022) Diplodia tip blight pathogen’s virulence empowered through host switch. Front Fungal Bio 3, article id 939007. https://doi.org/10.3389/ffunb.2022.939007.
Brodde L, Åslund MS, Elfstrand M, Oliva J, Wågström K, Stenlid J (2023) Diplodia sapinea as a contributing factor in the crown dieback of Scots pine (Pinus sylvestris) after a severe drought. For Ecol Manag 549, article id 121436. https://doi.org/10.1016/j.foreco.2023.121436.
Brookhouser LW, Peterson GW (1970) Infection of Austrian, Scots, and Ponderosa pines by Diplodia pinea. Phytopathology 61, article id 409. https://doi.org/10.1094/Phyto-61-409.
Bußkamp J (2018) Schadenserhebung, Kartierung und Charakterisierung des “Diplodia-Triebsterbens“ der Kiefer, insbesondere des endophytischen Vorkommens in den klimasensiblen Räumen und Identifikation von den in Kiefer (Pinus sylvestris) vorkommenden Endophyten. [Damage assessment, mapping and characterization of “Diplodia Tip Blight” of pines, especially the endophytic presence in climate-sensitive areas and identification of pine endophytes]. PhD thesis. Universität Kassel, Kassel.
Caballol M, Ridley M, Colangelo M, Valeriano C, Camarero JJ, Oliva J (2022) Tree mortality caused by Diplodia shoot blight on Pinus sylvestris and other mediterranean pines. For Ecol Manag 505, article id 119935. https://doi.org/10.1016/j.foreco.2021.119935.
Finnish Food Authority (2023) Siemen- ja taimitilastot 2006–2023. Ruokavirasto (Finnish Food Authority).
Flowers J, Nuckles E, Hartman J, Vaillancourt L (2001) Latent infection of Austrian and Scots pine tissues by Sphaeropsis sapinea. Plant Dis 10: 1107–1112. https://doi.org/10.1094/PDIS.2001.85.10.1107.
Keeling CI, Bohlmann J (2006) Diterpene resin acids in conifers. Phytochemistry 67: 2415–2423. https://doi.org/10.1016/j.phytochem.2006.08.019.
Li X, Liu B, Ren X, Cui J, Li H (2019) Infection approach of Diplodia sapinea, the causal agent of tip blight of Pinus tabulaeformis in China. For Path 49, article id e12544. https://doi.org/10.1111/efp.12544.
Milijašević T (2006) Effect of temperature on the mycelial growth of the fungus Sphaeropsis sapinea. Glasnik Šumarskog Fakulteta, 94: 211–222. https://doi.org/10.2298/GSF0694211M.
Muona O, Hiltunen R, Morén E, Shaw D (1986) Analysis of monoterpene variation in natural stands and plustrees of Pinus sylvestris in Finland. Silva Fenn 20: 1–8. https://doi.org/10.14214/sf.a15435.
Oostlander AG, Brodde L, von Bargen M, Leiterholt M, Trautmann D, Enderle R, Elfstrand M, Stenlid J, Fleißner A (2023) A reliable and simple method for the production of viable pycnidiospores of the pine pathogen Diplodia sapinea and a spore-based infection assay on Scots pine. Plant disease 107: 3370–3377. https://doi.org/10.1094/PDIS-01-23-0107-RE.
Perry A, Wachowiak W, Brown AV, Ennos RA, Cottrell JE, Cavers S (2016) Substantial heritable variation for susceptibility to Dothistroma septosporum within populations of native British Scots pine (Pinus sylvestris). Plant Pathol 65: 987–996. https://doi.org/10.1111/ppa.12528.
Persson T, Hall D, Barklund P, Samils B, Andersson Gull B (2024) The inheritance of resistance to Scots pine blister rust in Pinus sylvestris. For Ecol Manage 568, article id 122135. https://doi.org/10.1016/j.foreco.2024.122135.
Peterson GW (1977) Infection, epidemiology, and control of Diplodia blight of Austrian, Ponderosa, and Scots pines. Phytopathology 67: 511–514. https://doi.org/10.1094/Phyto-67-511.
Phillips AJ, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76: 51–167. https://doi.org/10.3114/sim0021.
Pyhäjärvi T, Kujala ST, Savolainen O (2019) 275 years of forestry meets genomics in Pinus sylvestris. Evol Appl 13: 11–30. https://doi.org/10.1111/eva.12809.
Quencez C, Bastien C (2001) Genetic variation within and between populations of Pinus sylvestris L. for susceptibility to Melampsora pinitorqua Rostr. (pine twist rust). Heredity 86: 36–44. https://doi.org/10.1046/j.1365-2540.2001.00800.x.
Rantanen M, Karpechko AY, Lipponen A, Nordling K, Hyvärinen O, Ruosteenoja K, Vihma T, Laaksonen A (2022) The Arctic has warmed nearly four times faster than the globe since 1979. Commun Earth Environ 3, article id 168. https://doi.org/10.1038/s43247-022-00498-3.
Rantanen M, Helama S, Räisänen J. Gregow H (2025) Summer 2024 in northern Fennoscandia was very likely the warmest in 2000 years. npj Clim Atmos Sci 8: 158. https://doi.org/10.1038/s41612-025-01046-4.
Semerci A, Semerci H, Çalişkan B, Çiçek N, Ekmekçi Y, Mencuccini M (2017) Morphological and physiological responses to drought stress of European provenances of Scots pine. Eur J Forest Res 136: 91–104. https://doi.org/10.1007/s10342-016-1011-6.
Stein Åslund M, Reichelt M, Zhang K, Castaño C, Stenlid J, Gershenzon J, Elfstrand M (2025) Scots pines with tolerance to Melampsora pinitorqua and Diplodia sapinea show distinct metabolic profiles. Plant Cell Environ 48: 1479–1493. https://doi.org/10.1111/pce.15218.
Taeger S, Zang C, Liesebach M, Schneck V, Menzel A (2013) Impact of climate and drought events on the growth of Scots pine (Pinus sylvestris L.) provenances. For Ecol Manage 307: 30–42. https://doi.org/10.1016/j.foreco.2013.06.053.
Terhonen E (2023) First report of Diplodia tip blight on Scots pine in Finland. Silva Fenn 56, article id 22008. https://doi.org/10.14214/sf.22008.
Terhonen E, Babalola J, Kasanen R, Jalkanen R, Blumenstein K (2021) Sphaeropsis sapinea found as symptomless endophyte in Finland. Silva Fenn 55, article id 10420. https://doi.org/10.14214/sf.10420.
Terhonen E, Ylioja T, Hytönen T, Leino K, Mutanen L, Melin M, Vaahtera E, Sutela S (2025) New saga in Finland: the rise of Diplodia sapinea in Scots pine. Fungal Genet Biol 176, article id 103955. https://doi.org/10.1016/j.fgb.2024.103955.
Yazdani R, Rudin D, Aldén T, Lindgren D, Harbom B, Ljung K (1982) Inheritance pattern of five monoterpenes in Scots pine (Pinus sylvestris L.). Hereditas 97: 261–272. https://doi.org/10.1111/j.1601-5223.1982.tb00879.x.
Total of 33 references.

