The colonization of young fire initiated stands by the crustose lichen Trapeliopsis granulosa and its potential effect on conifer establishment and stand succession
Splawinski T. B., Gauthier S., Fenton N. J., Houle D., Bergeron Y. (2018). The colonization of young fire initiated stands by the crustose lichen Trapeliopsis granulosa and its potential effect on conifer establishment and stand succession. Silva Fennica vol. 52 no. 1 article id 7791. https://doi.org/10.14214/sf.7791
Highlights
- T. granulosa is a poor seedbed for jack pine establishment
- The presence of extensive T. granulosa cover can limit ongoing tree recruitment, thereby maintaining open lichen woodland
- Dry open conditions favor the establishment of T. granulosa
- Stands with significant T. granulosa cover may be good candidates for afforestation initiatives due to lower evaporation potential and decreased water stress.
Abstract
The resilience of closed-crown coniferous stands within the boreal forest of North America is highly dependent on successful re-establishment of tree species following fire. A shift from closed-crown forest to open lichen woodland is possible following poor natural regeneration during the initial establishment phase, followed by the development of extensive lichen cover, which may hinder ongoing recruitment. We examined the development of the crustose lichen Trapeliopsis granulosa (Hoffm.) 18 to 21 years following fire within six sites in the boreal forest of northwestern Quebec, and explored its potential to affect ongoing recruitment during early successional stages of stand development. Germination and survivorship trials were conducted within the laboratory to determine the establishment rate of Pinus banksiana Lamb. (jack pine) on T. granulosa, mineral soil, and burnt duff under two separate watering frequencies (observed and drought). Survival and establishment rates of jack pine were highest on burnt duff, and poor on both T. granulosa and mineral soil. Under the drought treatment, no seedlings survived on any substrates. In the field, T. granulosa cover had a positive relationship with mineral soil cover, and negative relationships with duff cover, ericaceous shrub cover, organic layer depth, other lichen cover, and Sphagnum moss cover. No discernable relationship was found between T. granulosa and tree density, rock cover, dead wood cover or other moss cover. The development of extensive T. granulosa cover in fire-initiated stands can impede ongoing recruitment of conifer species due to its poor seedbed quality, thereby maintaining open forests.
Keywords
establishment;
Pinus banksiana;
forest ecology;
lichen woodland;
stand succession.;
Trapeliopsis granulosa
-
Splawinski,
Institut de recherche sur les forêts, Université du Québec en Abitibi-Témiscamingue, 445, boul. de l’Université, Rouyn-Noranda, QC, J9X 5E4, Canada
E-mail
tsplawinski@gmail.com

- Gauthier, Natural Resources Canada, Canadian Forest Service, Laurentian Forestry Centre, 1055 rue du PEPS, P.O. Box 10380, Stn Sainte Foy, QC, G1V 4C7, Canada E-mail sylvie.gauthier@rncan-nrcan.gc.ca
- Fenton, Institut de recherche sur les forêts (IRF), Université du Québec en Abitibi-Témiscamingue, 445 boul. de l’Université, Rouyn-Noranda, QC, J9X 5E4, Canada E-mail nicole.fenton@uqat.ca
- Houle, Ministère des Forêts, de la Faune et des Parcs, Direction de la recherché forestière, Québec, QC, G1P 3W8, Canada; Ouranos Climate Change Consortium, Montréal, QC, H3A 1B9, Canada E-mail daniel.houle@mffp.gouv.qc.ca
- Bergeron, Centre d’étude sur la forêt and Chaire industrielle en aménagement forestier durable, Université du Québec à Montréal, CP 8888 Succursale A, Montréal, QC, H3C 3P8, Canada E-mail bergeron.yves@uqam.ca
Received 22 August 2017 Accepted 12 January 2018 Published 17 January 2018
Views 169373
Available at https://doi.org/10.14214/sf.7791 | Download PDF

1 Introduction
The resilience of closed-crown coniferous stands within the boreal forest of North America is highly dependent on successful natural regeneration following fire (Coté et al. 2013). The two most common tree species within this biome, jack pine (Pinus banksiana Lamb.) and black spruce (Picea mariana (Mill.) Britton, Sterns & Poggenb.), are generally well adapted to this common, widespread and semi-random disturbance, with stands typically regenerating to a similar density as existed prior to disturbance, thereby maintaining a steady-state self-replacement dynamic over time (Johnstone et al. 2010; Greene and Johnson 1999). Intrinsically linked biotic and abiotic constraints can however limit re-establishment success, thereby altering the trajectory of succession (Johnstone et al. 2010). These are seed production and viability, seed predation, germination rate, fire severity, and temporal span between two successive disturbance events (Desjardins 2016; Cote et al. 2013; Pinno et al. 2013; Girard et al. 2008; Greene et al. 1999).
The recruitment period of jack pine and black spruce occurs within the first six years following fire, during which the vast majority of seeds from both species are abscised from the aerial seedbank (Greene et al. 2013). Most research on recruitment potential following disturbance has focused on pre-fire seed production and tree growth as a function of age, site productivity, and climate/latitude (Van Bogaert et al. 2015a,b; Greene and Johnson 1999; Rudolph and Laidly 1990; Viereck and Johnston 1990), and indirectly, seed viability as a function of crown fire severity (Pinno et al. 2013). Ground fire severity (depth of burn), which is generally controlled by fuel availability, soil moisture content, pre-fire organic layer depth, and the timing of fire occurrence within the fire season (Terrier et al. 2014, 2015; Greene et al. 2007, 2006; Miyanishi and Johnson 2002), will determine the availability of suitable seedbeds such as exposed mineral soil, a thin organic layer, or residual moss cover (Greene et al. 2006, 2004, 1999; Charron and Greene 2002; Greene and Johnson 1998, 1999; Sirois 1993). Water stress and extreme temperatures typical of recently burned areas will further limit tree recruitment through the desiccation of newly established individuals (Moss and Hermanutz 2009; Hogg and Hurdle 1995; Hogg 1994; Black and Bliss 1980; Viereck and Foote 1979).
Open lichen-spruce woodlands can be observed throughout the Canadian boreal forest (Rowe 1972). As described by Côté et al. (2013), Girard et al. (2008), Payette and Delwaide (2003), and Payette et al. (2000), a short time period between successive disturbance events can reduce the natural regeneration potential of conifer species, leading to an alternative stable state, marked by a shift from closed-crown forest to open lichen woodland, especially on well-drained soils. Although possible, reversion back to dense closed-crown forest has not been observed in the field (Girard et al. 2008; Jasinski et Payette 2005; Coxson and Marsh 2001; Kershaw 1977, 1978; Maikawa and Kershaw 1976). If reversion is achieved, it is the result of canopy expansion, and the subsequent replacement of lichens by feather mosses (Coxson and Marsh 2001; Morneau and Payette 1989; Foster 1985), since overhead cover will decrease light, thereby reducing lichen cover and increasing moisture (Root and McCune 2012; Coxson and Marsh 2001; Foster 1985; Kershaw 1977; Rouse and Kershaw 1971; Fraser 1956).
Little attention has been given to the development of crustose lichen mats following fire and their effects on ongoing natural recruitment during early successional stages of stand development. Continuous lichen cover has been shown to limit pine and spruce regeneration and growth, thereby maintaining open lichen-spruce woodlands (Pacé 2017; Houle and Filion 2003; Morneau and Payette 1989; Foster 1985; Cowles 1982; Hustich 1951; Ahti 1959; Allen 1929), however these studies focused on Cladonia spp. which dominate at a later successional stage. Lichen woodland communities are often concentrated on sandy well-drained soils (Jasinski et Payette 2005; Payette et al. 2000; Johnson and Miyanishi 1999; Oksanen and Ahti 1982; Kershaw 1977, 1978; Ahti 1959; Fraser 1956), where limited soil moisture and organic matter result in high surface temperatures. Such dry conditions are suitable for lichen colonization, but limit establishment and competition from other species (Sedia and Ehrenfeld 2003; Brulisaueret al. 1996; Kershaw 1977; Lambert and Maycock 1968).
The crustose lichen Trapeliopsis granulosa (Hoffm.) can form a continuous dense crust within the first twenty years following disturbance (Hugron et al. 2011; Morneau and Payette 1989). As observed by Morneau and Payette (1989), colonization of T. granulosa begins roughly 5 years following fire, with maximum cover occurring by 14 years. It is then gradually transitions to dominance by Cladonia spp. 25 years following fire, as observed by Morneau and Payette (1989) and Foster (1985), see also Kershaw (1977). T. granulosa is typically found on acidic soil, peat or rotting wood, including recently burned substrates, and is considered an important colonizer of bare soil and charred wood (McMullin and Anderson 2014; Brodo et al. 2001; Purvis et al. 1992; Foster 1985).
The objectives of this study are threefold: 1) to determine how the cover of T. granulosa is influenced by biotic and abiotic site characteristics; 2) in a laboratory experiment, to determine the establishment rate of jack pine on samples of the crustose lichen T. granulosa, and compare it to establishment on mineral soil and burnt duff; and 3) to discuss how its presence may help maintain open lichen woodlands in the Canadian boreal forest.
2 Methodology
2.1 Study area
Our study area was located in the Lake Matagami lowland ecological region (6a), situated in the western portion of the spruce-moss bioclimatic domain within the managed continuous boreal forest of western Quebec (Canada). This region is characterized by a sub-polar sub-humid continental climate, with total annual precipitation ~825 mm, and an average annual temperature of 0 °C at the southern limit. Growing season length is approximately four to five months (Bergeron et al. 1998). Typical surficial deposits include glaciolacustrine clays and sands, glacial tills, glacio-fluvial complexes, and organic surficial deposits (Bergeron et al. 1998; Blouin and Berger 2005).
We examined sites found within three forest fires occurring between 1995 and 1998 (Fig. 1), situated along the boundary between the southern limit of the western spruce-moss and northern limit of the western balsam fir-white birch bioclimatic domains (Bergeron et al. 1998; Blouin and Berger 2005).
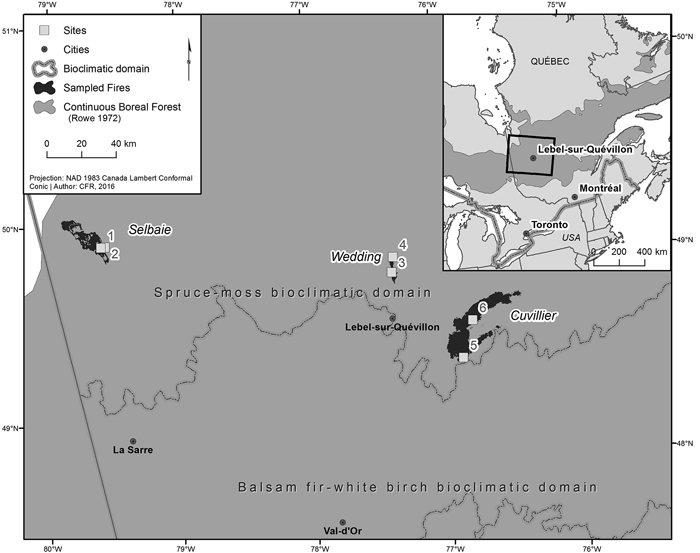
Fig. 1. Location of the three fires and associated sites sampled within the western portion of the spruce-moss bioclimatic domain, in the managed continuous boreal forest of western Quebec.
The Selbaie fire was located in northwestern Quebec 100 km north of the town of La Sarre (49°58´N, 79°18´W). Ignited by lightning on June 6 1997, it burned 18 319 ha of forest before being extinguished by rain on July 8. The Wedding fire was located in northwestern Quebec 21 km north of the town of Lebel-sur-Quevillon (49°13´N, 76°53´W). Ignited accidentally on May 16 1998, it burned 4130 ha of forest before being extinguished by rain on June 16. The Cuvillier fire was located in 36 km east of the town of Lebel-sur-Quevillon (48°48´N, 76°35´W). Ignited accidentally on August 16 1995, it burned 47 709 ha of forest before being extinguished by rain on October 20.
Two sites per fire, for a total of 6 sites dominated by black spruce and/or jack pine were sampled in the summer of 2016 within these three burned areas, with site selection limited to those areas where T. granulosa was present. Within all sampled sites, 100% tree mortality occurred as a result of fire, and five of the six sites were salvage logged. Additional site details can be found in Tables 1 and 2. The dominant lichen was T. granulosa, a verrucose lichen with a greenish white to pale gray thallus (Fig. 2). Aphothecia ranged from pale pinkish brown to black and usually had persisting margins, though they can become very thin or disappear with time. T. granulosa was growing over charcoal and/or burnt moss, burnt wood pieces, and mineral soil (Fig. 3). Other Cladonia spp. lichens were present, including C. stellaris and C. rangiferina. Commonly observed mosses included Polytrichum spp. and Dicranum spp., and to a lesser extent, Ptilium crista-castrensis (Hedw.) De Not., Hylocomium splendens (Hedw.) Schimp., and Pleurozium schreberi (Brid.) Mitt.. Sphagnum spp. were occasionally observed in depressions in mesic sites. Ericaceous shrubs included the species Vaccinium spp., Kalmia angustifolia L., Ledum groenlandicum Oeder. Lichen species were identified using both a visual and chemical approach based on the nomenclature of McMullin and Anderson (2014) at the Forest Research Institute (Université du Québec en Abitibi-Témiscamingue) under a stereomicroscope (Olympus model SZX12, magnification 8.4X.). Information from Brodo et al. (2001) was used for secondary confirmation.
| Table 1. Site details including fire information, species composition, drainage, surficial deposit, and forest management type. BS = black spruce, JP = jack pine, TSF = time since fire. | |||||||
| Site | Fire name and year | TSF | Dominant species composition | Drainage | Surficial deposit | Salvage logging | Planting |
| 1 (xeric) | Selbaie (1997) | 19 | BS | Well drained | Sand | Yes | Yes |
| 2 (xeric) | Selbaie (1997) | 19 | BS | Well drained | Sand | Yes | Yes |
| 3 (xeric) | Wedding (1998) | 18 | BS | Moderate – Well drained | Sand | Yes | No |
| 4 (highly xeric) | Wedding (1998) | 18 | BS/JP | Excessive | Sand | No | Yes |
| 5 (xeric) | Cuvillier (1995) | 21 | BS/JP | Well drained | Sand | Yes | No |
| 6 (mesic) | Cuvillier (1995) | 21 | BS/JP | Moderate | Sand | Yes | No |
| Table 2. Site details including tree stocking and density, mean organic layer depth, and mean understory cover. Tg = Trapeliopsis granulosa cover and thickness. | ||||||||||
| Site | Tree stocking | Tree density (stems ha–1) | Organic layer depth (cm) | Tg cover (%) | Tg thickness (cm) | Other lichen cover (%) | Total lichen cover (%) | Moss (%) | Sphagnum moss (%) | Ericaceous shrub cover (%) |
| 1 | 60 | 1750 | 9 | 55 | 1.2 | 21 | 76 | 2.1 | 0 | 35 |
| 2 | 80 | 2500 | 8 | 35 | 1.2 | 29 | 64 | 9.7 | 0.3 | 20 |
| 3 | 10 | 250 | 7 | 17 | 1.3 | 39 | 56 | 3.6 | 0 | 62 |
| 4 | 80 | 2250 | 4 | 63 | 1.3 | 13 | 76 | 2.4 | 0 | 36 |
| 5 | 30 | 1000 | 9 | 27 | 1.1 | 40 | 67 | 3.6 | 0.4 | 58 |
| 6 | 40 | 4000 | 14 | 10 | 1.2 | 54 | 64 | 4.6 | 3.8 | 66 |

Fig. 2. Trapeliopsis granulosa as seen through a microscope, magnification 8.4X.

Fig. 3. Example of Trapeliopsis granulosa on burnt duff from site 1. Kalmia angustifolia flowers are visible in the image on the left.
Within each site we established a single randomly located transect composed of ten 4 m2 circular micro-plots spaced 10 m apart. Within each micro-plot we recorded the stem density of black spruce and jack pine (to determine stand density and stocking), and the percent cover of the forest floor in the following categories: T. granulosa, other lichen species, ericaceous species, feather mosses, Sphagnum mosses, dead wood, duff, and exposed mineral soil. The sum of these different categories could exceed 100%, since some were superimposed on others. Additionally, one measurement of the thickness of T. granulosa was recorded at the closest point to center of each micro-plot, as well as the depth of the organic layer to the underlying surficial deposit, measured at the center of each micro-plot. The surficial deposit type, mean organic layer depth, and indicator plant species were then used to determine site drainage, which ranged from moderate to excessive.
We removed four 1 m2 samples of T. granulosa and 100 kg of mineral soil from four of the six sites (Sites 1,3,5,6) representing the three fires, to be used in germination trials in the laboratory. T. granulosa samples were underlain by either burnt duff or mineral soil. In site 1, where burnt duff was present under all samples, we carefully removed the overlying T. granulosa and then also removed the duff itself. The burnt duff had a thickness of 6–9 cm. The mineral soil from all 4 sites was sample to a depth of 30 cm and then mixed together in the laboratory.
Jack pine germination and survivorship trials were conducted in the greenhouse at the Université du Québec à Montréal under controlled temperature, light, and humidity levels over a period of three months, reflecting the prime recruitment period of the year (June–August) for conifer species within our study area.
A total of 40 6-inch deep (24 × 36 inch) terraria were used for the experiment. Each terraria had holes drilled into the bottom, followed by a sheet of weed-barrier, and 3 inches of mixed mineral soil taken from the sites to allow for adequate drainage. Lichen samples obtained from each of the four sites were divided into eight 0.5 m2 sections, yielding a total of 32 sections. Each section was then placed on top of the mineral soil in the terraria. This process was repeated for the burnt duff samples removed from site 1, yielding four sections. A mineral soil only treatment was also included, yielding the final four sections. Consequently there were 40 terraria: 32 with lichen, four with burnt duff and four with mineral soil.
Jack pine seeds were obtained from the Berthier forestry seed center, Ministère des Forêts, de la Faune et des Parcs branch of the Quebec provincial government. They had a laboratory tested germination rate of 96%. Using a dial seed sower, 20 seeds were sown at a minimum spacing of 3 cm directly on the substrate in each terrarium (crust, burnt duff, or mineral soil). Seeds were placed on the surface of each substrate in order to reflect the conditions of natural dispersal.
The influence of two separate watering regimes on seed germination and survivorship was tested. Rainfall and temperature data was obtained from the Val d’Or weather station, located at a mean distance of 153 km from all three fires sampled (115 km Min., 200 km Max.). This weather station was selected because it was the closest with a continuous record of data for the 30-year reference period, and was therefore used as a proxy for climate within our study area. Daily maximum, minimum, and mean temperature, as well as the mean precipitation amount per rainfall event, and mean days between rainfall events were calculated for the months of June to August (Table 3). This time period was selected to reflect the typical natural germination period of small-seeded conifers such as jack pine (Splawinski et al. 2014; Gauthier et al. 1993).
| Table 3. Daily maximum, minimum, and mean temperature, as well as the mean precipitation amount per rainfall event, and mean days between rainfall events observed at the Val d’Or weather station between June and August (1986–2016). | ||||
| Val d’Or data (1986–2016) | Mean | Standard deviation | Lower 95% Cl | Upper 95% Cl |
| Maximum temperature (°C) | 22.57 | 4.73 | 22.39 | 22.74 |
| Minimum temperature (°C) | 10.05 | 4.44 | 9.89 | 10.22 |
| Mean temperature (°C) | 16.33 | 4.11 | 16.18 | 16.48 |
| Total precipitation (mm) | 5.39 | 7.95 | 4.99 | 5.78 |
| Days between rain events | 2.56 | 2.36 | 2.42 | 2.70 |
Half of the terraria (i.e. four lichen terraria representing each of the four sites (16 total), two burnt duff, and two mineral soil terraria) were subjected to a “baseline” watering treatment, representing mean precipitation frequency and amount observed at the Val d’Or weather station over the 1986–2016 reference period (2.6 days between watering events and 5.4 mm per watering), while the other half was subjected to the “drought” treatment, where we doubled the period between rain events (5.2 days) but maintained the same amount per rain event (5.4 mm) (Table 3).
The temperature within the greenhouse was changed four times a day for a period of six hours. We used the mean maximum temperature as the daily high (23 °C), and the mean average temperature (16 °C) as the daily low for both the baseline and drought treatments. Humidity was maintained at a constant rate (60%).
Lighting was provided by GE Lucalox 400 Watt Electronic Bulbs (LU750/400 PLS/T40 400, Nominal lumen = 48 000), suspended from the ceiling, approximately ten feet above the terraria. The lights were set on a timer to provide 12 hours of light daily from 7 AM until 7 PM.
All terraria were monitored for germination twice a week over a period of three months, and to ensure proper control measures. At the end of the three-month experiment, the total number of surviving seedlings was determined for each terrarium, thus providing us with an indicator of establishment potential.
2.2 Statistical analyses
Principle component analysis (PCA) was used to visualize the relationship between T. granulosa cover, other lichen cover, ericaceous shrub cover, moss cover, mineral soil cover, dead wood cover, duff cover, rock cover, organic layer depth, and tree density. The difference in T. granulosa thickness between sites was tested with an ANOVA.
To study the effect of substrate type (mineral soil, burnt duff, and lichen from each of the four sites sampled) on the survivorship frequency we used a frequency table analysis (Chi2). Survivorship data from the four replicate terraria of T. granulosa were lumped for each site, as were the two replicate terraria (each) of burnt duff and mineral soil. A α-value of 0.05 was used for all statistical analyses.
3 Results
3.1 Field survey data
T. granulosa had a positive relationship with mineral soil cover, and a negative relationships with duff cover, ericaceous shrub cover, organic layer depth, other lichen cover, and Sphagnum moss cover as indicated by the principal component analysis. There was no discernable relationship between T. granulosa and tree density, rock cover, dead wood cover or other moss cover. The first axis explains 28.37% of the total variance, and the second explains 15.39%, for a total of 43.77% (Fig. 4). No significant differences in T. granulosa lichen thickness (R2 = 0.17; F-value = 0.0607) were observed between sites.
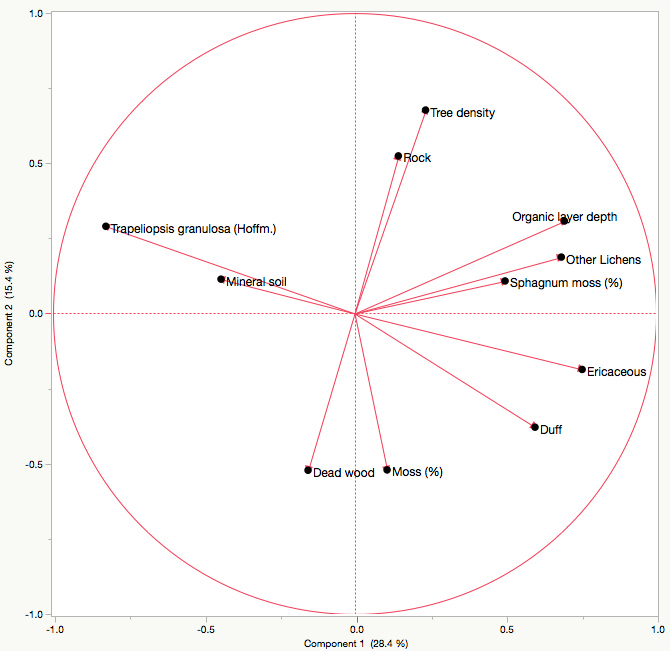
Fig. 4. Principal component analysis illustrating the relationship between Trapeliopsis granulosa cover, other lichen cover, ericaceous shrub cover, moss cover, mineral soil cover, dead wood cover, duff cover, rock cover, organic layer depth, and tree density. Axes 1 and 2 have a combined explanation power of 43.77%.
3.2 Jack pine germination and survivorship trials
Germination began within the first week, with most germinants having emerged by the end of the second week. Following the three-month trial period, jack pine survival was highest on burnt duff with no significant differences observed among the other substrates (Fig. 5). Under the drought treatment, no seedlings survived on any of the substrates (mineral soil, burnt duff, lichen) at the end of the three-months trial period (not shown).
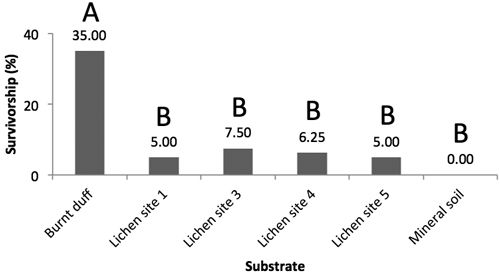
Fig. 5. Mean jack pine seedling survival rates (%) on burnt duff, Trapeliopsis granulosa from site 1, 3, 4, and 5, and mineral soil (Prob. > Chi2 < 0.0001). Survival data for each substrate has been lumped. Letters illustrate significant differences in survival rates between substrates.
4 Discussion
4.1 Abundance of Trapeliopsis granulosa
Lichen species most often colonize well-drained upland sites with thin organic layers where exposed mineral soil tends to be common following fire (Roturier et al. 2007; Jasinski and Payette 2005; Payette et al. 2000). For most lichen species, establishment will occur within the first five to fifteen years following fire (Morneau and Payette 1989). However, the absence of T. granulosa and other lichen species in young stands does not necessarily imply poor establishment conditions, but could be the result of a lack of spores or algal partner. Therefore, we limited site selection to those that contained varying degrees of T. granulosa cover (Table 2) in order to assess factors influencing its abundance. The PCA indicated that T. granulosa abundance was positively correlated with exposed mineral soil cover. Conversely, negative relationships were observed with organic layer depth and Sphagnum moss cover, which is not surprising given that an increase in both of these factors is linked to poorer site drainage. No discernable relationship between T. granulosa and rock or dead wood cover was observed, substrates that this lichen species is able to colonize.
Direct competition for resources and space from other lichen species and ericaceous shrubs appeared to limit T. granulosa abundance. The latter has an advantage since asexual regeneration and growth occurs rapidly following natural disturbance, especially in open conditions (Morneau and Payette 1989; Bunnell 1990). Ericaceous shrubs therefore have the capacity to not only limit resources and space, but light availability as well. Similarly, the accumulation of duff from shed leaves and other organic material inhibit lichen abundance, as indicated by the PCA.
Lichen cover tends to be inversely proportional to tree density, since forest cover significantly reduces light availability while promoting moss establishment due to increased moisture (Boudreault et al. 2013; Root and McCune 2012; Coxson and Marsh 2001; Foster 1985; Kershaw 1977; Rouse and Kershaw 1971). Jack pine can exhibit very high natural regeneration densities following fire (Bella 1974; De Groot et al. 2004), reaching up to 200 000 stems ha–1 (Splawinski et al. Unpublished), however densities observed in the field are highly variable. From a forest management perspective, a natural seedling density of roughly 1 stem m–2 (10 000 stems ha–1) is considered adequate to fully re-stock a stand (Splawinski et al. 2014; Greene et al., 2002). When natural regeneration is insufficient, conifer plantations are typically established at a density of 2000 stems/ha (Thiffault et al. 2013, 2003).
We did not observe a relationship between tree density and T. granulosa abundance. In all of our sampled sites, total lichen cover (all species lumped together) was high, ranging from 56 to 76% (Table 2). Stand stocking was highest in planted sites, but overall tree densities from 5 of the 6 sites were relatively low (250–2500 stems ha–1). The exception was site 6, which had a tree density of 4000 stems ha–1 (Table 2), however most of these stems did not originate from the establishment phase following fire, but through subsequent recruitment from sexually mature individuals originating from the first cohort, and less likely, through long distance dispersal from an adjacent stand. Interestingly, not one of these recruits was found on lichen. Instead, they were primarily concentrated on living sphagnum and to a lesser extent on duff. Although we did not measure tree height, which is mainly influenced by site productivity (Rudolph and Laidly 1990; Viereck and Johnston 1990), ample sunlight was available in all sites throughout the understory due to wide spacing between trees and small crowns.
Our sites were sampled between 18 and 21 years following fire (Table 1). According to the lichen-moss successional stages outlined by Coxson and Marsh (2001), Morneau and Payette (1989), and Foster (1985), these stands are still in the earliest stage (approximately <25 years), with the surface dominated by crustose lichens and moss. The presence of young Cladonia spp. and to a far lesser extent, reindeer lichen indicates that progression to next stage has begun. Morneau and Payette (1989) observed rapid expansion by lichens and ericaceous shrubs following fire. Similar to their results (at 23 years following fire), all of our well and excessively drained sites were dominated by lichens, followed by ericaceous shrubs. Our moderate to well-drained sites (sites 3 and 6) had almost equal cover of lichens and shrubs.
4.2 Establishment trials
Under the baseline scenario, burnt duff was found to be the best seedbed, whereas T. granulosa and mineral soil were very poor. Although we expected low establishment potential on lichen, the mineral soil results were unexpected as mineral soil is typically considered a good quality seedbed for the establishment of small-seeded conifer species such as jack pine and black spruce (Greene et al. 2004; Charron and Greene 2002; Greene et al. 1999). We believe that this may be due to two reasons: 1) that mineral soil and burnt duff characteristics have changed over the 18–21 years since fire, and may have been further altered due to the development of overlying lichen cover, and 2) due to the thermal and moisture conditions within the greenhouse itself, an argument also proposed by Pacé (2017), who likewise observed higher germination and survival on lichen (Cladonia spp.) than mineral soil.
Direct unfiltered light 12 hours daily, and a short (5 minutes) but heavy (5.4 mm) watering period may have led to higher rates of evaporation and drainage on exposed mineral soil due to a lack of overlying cover (duff or lichen). Although not directly quantified, almost all seeds on mineral soil under the baseline scenario germinated, however each succumbed to desiccation shortly after. Thus, there was adequate moisture to stimulate germination, but not enough to ensure survival.
Although likely due to the above-mentioned factors, these results indicate that mineral soil may not always be a good seedbed, especially if it is found in xeric sites with excessive drainage. Indeed, Gagnon (1966) observed significantly greater germination of black spruce on the crustose lichen Lecidea granulosa (Enrh.) versus on mineral soil, which was attributed to the water retention capacity of lichen cover. If established early enough, it was argued that this lichen might improve establishment of black spruce (Kershaw 1977; Gagnon 1996). Although we did not observe significantly greater establishment on T. granulosa than on mineral soil, the former was higher (5%–7.5%) than the latter (0%). Under projected climate change, xeric sites may become more susceptible to regeneration failure due to prolonged and more frequent drought (Peng et al. 2011). Our results from the drought treatment, where no survivorship was observed on any substrate, provide further proof that water stress plays a primary role in initial establishment (Moss and Hermanutz 2009).
During germination trials, although the upper surface of T. granulosa dried quickly, the underlying mineral soil remained moist for extended periods of time, a phenomenon also observed by Fraser (1956) on Cladonia mats in Quebec (see also Kershaw 1977), and Pacé (2017) in greenhouse experiments. We believe that the upper surface of T. granulosa inhibited germination by drying rapidly, and presented a barrier to penetration by those individuals that were able to germinate, leading to desiccation before the radicle could reach the underlying moist mineral soil (Foster 1985; Allen 1929). Another inhibitory effect, allelopathy, has been shown to limit germination (Ramault and Corvisier 1975), however this was not examined in our study.
It has been suggested that lichen mats may potentially be good substrates for establishment (Kershaw 1977) if seeds fall within cracks between lichen cover (Sirois 1993). Interestingly, although no surviving individuals were recorded following the trial period under the drought treatment, two seeds that fell off one of the crust samples and onto the underlying mineral soil when sown, germinated and survived through to the end of the trial period. This was certainly due to moisture retention and shade in the underlying soil.
4.3 The role of lichens in limiting ongoing recruitment
Low initial tree densities explain how closed forests could transition to open lichen woodlands, but do not explain how they are maintained in the long-term. Lichen colonization typically begins 5 to 10 years following fire (Hugron et al. 2011; Morneau and Payette 1998). Therefore it could not explain the initial poor recruitment of conifers following fire, since the recruitment period of black spruce and jack pine is typically limited to the first 6 years following disturbance, when the vast majority of seed abscission occurs in both species (Splawinski et al. 2014; Greene et al. 2013). Poor initial recruitment densities would most likely be the result of a combination of factors, including poor seed production due to the age of pre-fire trees or site productivity, poor seed viability due to high fire severity, or a low germination rate due to a lack of suitable seedbeds and water stress.
Ongoing recruitment beyond the initial post-fire establishment phase is possible once jack pine and black spruce attain sexual maturity (Gauthier et al. 1993; Viereck and Johnston 1990; Morneau and Payette 1989; Foster 1985). Long distance dispersal from adjacent mature stands can also potentially improve stand density, but it declines rapidly with distance from seed source (Greene and Johnson 1996, 1993, 1989; Foster 1985). However, the establishment of extensive lichen cover and exclusion of suitable microsites could effectively limit any such ongoing recruitment (Houle and Filion 2003; Morneau and Payette 1989; Foster 1985; Cowles 1982; Fisher 1980, 1979; Hustich 1951; Ahti 1959), thereby maintaining low stand density. In regions with short fire cycles and/or dry upland sites, low stand densities could translate into the long-term maintenance of open lichen woodlands (Morneau and Payette 1989).
4.4 Management implications
Afforestation of open lichen woodlands has yielded positive results (Côté et al. 2014; Hébert et al. 2014; Tremblay et al. 2013), and may be a useful management tool in reverting forests back to closed cover. As pointed out by Rapanoela et al. (2015) and Van Bogaert et al. (2015b) however, it may not be a suitable option for northern boreal woodlands due to climatic constraints on tree growth and fire risks.
Although a relatively poor seedbed for germination, extensive stand cover of T. granulosa and other crustose lichen species may reduce the effects of drought for established individuals by maintaining soil moisture for a prolonged period of time (Kershaw 1977). This may make plantations a viable option for the afforestation of open lichen forests, since individuals would be planted directly into the underlying mineral soil, which would be especially beneficial in excessively or well-drained sites where water stress is common.
Under typical plantation densities, eventual canopy expansion and closing would shift ground cover from lichen to moss (Morneau and Payette 1989). Tree growth rates, however, may be reduced by the presence of significant lichen cover due to allelopathy, reduced nutrient availability, changes in the root ectomycorrhizal community, and cooling of soil beneath the lichen mat (Pacé 2017; Foster 1985; Arseneault 1979; Fisher 1979; Kershaw 1977; Brown and Mikola 1974). This may extend the time period necessary to attain canopy closure (Kershaw 1977), thereby delaying the return to closed forests, which may prove problematic in regions under a short fire cycle. It is important to note however, that these studies focused on Cladonia spp. only, which tend to appear at a later in stand development (Coxson and Marsh 2001; Morneau and Payette 1989; Foster 1985). In sampled sites with planted stock, we observed modest tree growth. These plantations were established 1–2 years following fire, and were therefore presumably able to develop sufficiently prior to widespread lichen colonization.
More research is required on the establishment dynamics of T. granulosa following fire and its impact on tree recruitment prior to extensive colonization by Cladonia spp. Potential inhibitory effects on tree growth due to extensive T. granulosa cover, similar to that observed in Cladonia spp., should also be assessed. In order to currently avoid such risks, a proactive approach can be taken, where sites with poor regeneration following fire and at risk of being colonized by extensive lichen cover are identified and planted quickly following disturbance.
5 Conclusion
Survival and establishment rates of jack pine were low on all substrates examined under the baseline treatment. Burnt duff engendered the highest germination and survival rates, significantly different from both T. granulosa and mineral soil. Under the drought treatment, no seedlings survived on any substrates. In the field, T. granulosa cover had a positive relationship with mineral soil cover, and negative relationships with duff cover, ericaceous shrub cover, organic layer depth, other lichen cover, and Sphagnum moss cover. The development of crustose lichen mats (here T. granulosa) in fire-initiated stands is facilitated by open and dry conditions. Extensive lichen cover has the potential to hinder ongoing recruitment of conifer species due to its poor seedbed quality, thereby contributing to the maintenance open forests.
Acknowledgments
We thank Mélanie Desrochers for GIS and mapping support, Stéphane Daigle for statistics support, and Julie Arseneault for lichen identification. Funding was provided by a MITACS Accelerate fellowship. Antoine Plouffe Leboeuf assisted in the field.
References
Ahti R. (1959). Studies on the caribou lichen stands of Newfoundland. Annales Botanici Societas ‘Vanamo’ 30: 1–44.
Allen A.E. (1929). Influence of Cladonia ground cover on the establishment of seedlings. Ecology 10(3): 354–355. https://doi.org/10.2307/1929515.
Amiro B.D., Stocks B.J., Alexander M.E., Flanniagn M.D., Wotton B.M. (2001). Fire, climate change, carbon and fuel management in the Canadian boreal forest. International Journal of Wildland Fire 10(4): 405–413. https://doi.org/10.1071/WF01038.
Bella I.E. (1974). Growth response of young jack pine to mechanical strip thinning. Manitoba Department of Environment, Canadian Forest Service, Edmonton, AB. Information Report NOR-X102. 11 p.
Bergeron J.-F., Grondin P., Blouin J. (1998). Rapport de classification écologique du sous-domaine bioclimatique de la pessière à mousses de l’ouest. [Ecological classification report of the western spruce-moss bioclimatic subdomain]. Ministère des Ressources naturelles du Québec, Direction des inventaires forestiers. 204 p.
Bergeron Y., Cyr D., Girardin M.P., Carcaillet C. (2010). Will climate change drive 21st century burn rates in Canadian boreal forest outside of its natural variability: Collating global climate model experiments with sedimentary charcoal data. International Journal of Wildland Fire 19(8): 1127–1139. https://doi.org/10.1071/WF09092.
Black R.A., Bliss L.C. (1980). Reproductive ecology of Picea mariana (Mill.) BSP., at tree line near Inuvik, Northwest Territories, Canada. Ecological Monographs 50(3): 331–354. https://doi.org/10.2307/2937255.
Blouin J., Berger J.-P. (2005). Guide de reconnaissance des types écologiques de la région écologique 6a – Plaine du lac Matagami et 6b – Plaine de la baie de Rupert. [Ecological type recognition guide for ecological region 6a – Lake Matagami plain and 6b – Rupert Bay plain]. Ministère des Ressources Naturelles et de la Faune, Direction des inventaires forestiers, Division de la classification écologique et productivité des station.
Boudreault C., Zouaoui S., Drapeau P., Bergeron Y., Stevenson S. (2013). Canopy openings created by partial cutting increase growth rates and maintain the cover of three Cladonia species in the Canadian boreal forest. Forest Ecology and Management 304: 473–481. https://doi.org/10.1016/j.foreco.2013.05.043.
Boulanger Y., Gauthier S., Burton P.J. (2014). A refinement of models projecting future Canadian fire regimes using homogeneous fire regime zones. Canadian Journal of Forest Research 44(4): 365–376. https://doi.org/10.1139/cjfr-2013-0372.
Brodo I.M., Sharnoff S.D., Sharnoff S. (2001). Lichens of North America. Yale University Press, USA. 795 p.
Brown R.T., Mikola P. (1974). The influence of fruticose soil lichens upon mycorrhizae and seedling growth of forest trees. Acta Forestalia Fennica 141: 1–20. https://doi.org/10.14214/aff.7575.
Brulisauer A.R., Bradfield G.E., Maze J. (1996). Quantifying organizational change after fire in lodgepole pine forest understorey. Canadian Journal of Botany 74(11): 1773–1782. https://doi.org/10.1139/b96-214.
Bunnell F.L. (1990). Reproduction of salal (Gaultheria shallon) under forest canopy. Canadian Journal of Forest Research 20(1): 91–100. https://doi.org/10.1139/x90-013.
Charron I., Greene D.F. (2002). Post-wildfire seedbeds and tree establishment in the southern mixedwood boreal forest. Canadian Journal of Forest Research 32(9) 1607–1615. https://doi.org/10.1139/x02-085.
Côté D., Girard F., Hebert F., Bouchard S., Gagnon R., Lord D. (2013). Is the closed-crown boreal forest resilient after successive stand disturbances? A quantitative demonstration from a case study. Journal of Vegetation Science 24(4): 664–674. https://doi.org/10.1111/j.1654-1103.2012.01488.x.
Côté D., Lupi C., Gagnon R., Lord D., Morin H. (2014). Growth dynamics of successive post-fire cohorts of black spruce: Is site potential reduced? The Forestry Chronicle 90(1): 96–104. https://doi.org/10.5558/tfc2014-015.
Cowles S. (1982). Preliminary results investigating the effect of lichen ground cover on the growth of black spruce. Naturaliste Canadien 109: 573–581.
Coxson D.S., Marsh J. (2001). Lichen chronosequences (postfire and postharvest) in lodgepole pine (Pinus contorta) forests of northern interior British Columbia. Canadian Journal of Botany 79(12): 1449–1464. https://doi.org/10.1139/b01-127.
De Groot W.J., Bothwell P.M., Taylor S.W., Wotton B.M., Stocks B.J., Alexander M.E. (2004). Jack pine regeneration and crown fires. Canadian Journal of Forest Research 34(8): 1634–1641. https://doi.org/10.1139/x04-073.
Desjardins F. (2016). Impact des insectes du sol sur la disponibilité des graines de pin gris et d’épinette noire après le passage du feu. [Impact of soil insects on jack pine and black spruce seed availability following fire]. Mémoire, Maîtrise en sciences forestières, Université Laval, Québec, Canada. 89 p.
Flannigan M., Logan K.A., Amiro B.D., Skinner W.R., Stocks B.J. (2005). Future area burned in Canada. Climatic Change 72(1–2): 1–16. https://doi.org/10.1007/s10584-005-5935-y.
Flannigan M.D., Krawchuk M.A., De Groot W.J., Wotton B.M., Gowman L.M. (2009). Implications of changing climate for global wildland fire. International Journal of Wildland Fire 18(5): 483–507. https://doi.org/10.1071/WF08187.
Fisher R.F. (1979). Possible allelopathic effects of reindeer-moss (Cladonia) on jack pine and white spruce. Forest Science 25: 256–260.
Fisher R.F. (1980). Allelopathy: a potential cause of regeneration failure. Journal of Forestry 78: 346–350.
Foster D.R. (1985). Vegetation development following fire in Picea mariana (black spruce) – Pleurozium forests of south-eastern Labrador, Canada. The Journal of Ecology 73(2): 517–534. https://doi.org/10.2307/2260491.
Fraser E.M. (1956). The lichen woodlands of the Knob Lake area of Quebec-Labrador. McGill Subarctic Research Papers 1: 3–28.
Gagnon J.D. (1996). Le lichen Lecidea granulosa constitue un milieu favorable à la germination de l’épinette noire. [The lichen Lecidea granulosa, a favorable seedbed for the germination of Picea mariana]. Naturaliste Canadien 93(2): 89–98.
Gauthier S., Bergeron Y., Simon J.-P. (1993). Cone serotiny in jack pine: ontogenetic, positional, and environmental effects. Canadian Journal of Forest Research 23(3): 394–401. https://doi.org/10.1139/x93-057.
Gauthier S., Raulier F., Ouzennou H., Saucier J.P. (2015). Strategic analysis of forest vulnerability to risk related to fire: an example from the coniferous boreal forest of Quebec. Canadian Journal of Forest Research 45(5): 553–565. https://doi.org/10.1139/cjfr-2014-0125.
Girard F., Payette S., Gagnon R. (2008). Rapid expansion of lichen woodlands within the closed-crown boreal forest zone over the last 50 years caused by stand disturbances in eastern Canada. Journal of Biogeography 35(3): 529–537. https://doi.org/10.1111/j.1365-2699.2007.01816.x.
Girard F., Payette S., Gagnon R. (2009). Origin of the lichen-spruce woodland in the closed-crown forest zone of eastern Canada. Global Ecology and Biogeography 18(3): 291–303. https://doi.org/10.1111/j.1466-8238.2009.00449.x.
Greene D.F., Johnson E.A. (1989). A model of wind dispersal of winged or plumed seeds. Ecology 70(2): 330–347. https://doi.org/10.2307/1937538.
Greene D.F., Johnson E.A. (1993). Seed mass and dispersal capacity in wind-dispersed diaspores. Oikos 67(1): 69–74. https://doi.org/10.2307/3545096.
Greene D.F., Johnson E.A. (1996). Wind dispersal of seeds from a forest into a clearing. Ecology 77(2): 595–609. https://doi.org/10.2307/2265633.
Greene D.F., Johnson E.A. (1998). Seed mass and early survivorship of tree species in upland clearings and shelterwoods. Canadian Journal of Forest Research 28(9): 1307–1316. https://doi.org/10.1139/x98-106.
Greene D.F., Johnson E.A. (1999). Modelling recruitment of Populus tremuloides, Pinus banksiana, and Picea mariana following fire in the mixedwood boreal forest of central Saskatchewan. Canadian Journal of Forest Research 29(4): 462–473. https://doi.org/10.1139/x98-211.
Greene D.F., Zasada J.C., Sirois L., Kneeshaw D., Morin H., Charron I., Simard M.-J. (1999). A review of the regeneration dynamics of North American boreal forest tree species. Canadian Journal of Forest Research 29(6): 824–839. https://doi.org/10.1139/x98-112.
Greene D.F., Kneeshaw D.D., Messier C., Lieffers V., Cormier D., Doucet R., Coates K.D., Groot A., Grover G., Calogeropoulos C. (2002). Modelling silvicultural alternatives for conifer regeneration in boreal mixedwood stands (aspen/white spruce/balsam fir). Forestry Chronicle 78(2): 281–295. https://doi.org/10.5558/tfc78281-2.
Greene D.F., Noel J., Bergeron Y., Rousseau M., Gauthier S. (2004). Recruitment of Picea mariana, Pinus banksiana, and Populus tremuloides across a burn severity gradient following wildfire in the southern boreal forest of Quebec. Canadian Journal of Forest Research 34(9): 1845–57. https://doi.org/10.1139/x04-059.
Greene D.F., Gauthier S., Noël J., Rousseau M., Bergeron Y. (2006). A field experiment to determine the effect of post‐fire salvage on seedbeds and tree regeneration. Frontiers in Ecology and the Environment 4(2): 69–74. https://doi.org/10.1890/1540-9295(2006)004[0069:AFETDT]2.0.CO;2.
Greene D.F., Macdonald S.E., Haeussler S., Domenicano J.N., Jayen K., Charron I., Gauthier S., Hunt S., Gielau E.T., Bergeron Y., Swift L. (2007). The reduction of organic-layer depth by wildfire in the North American boreal forest and its effect on tree recruitment by seed. Canadian Journal of Forest Research 37(6): 1012–1023. https://doi.org/10.1139/X06-245.
Greene D.F., Splawinski T.B., Gauthier S., Bergeron Y. (2013). Seed abscission schedules and the timing of post-fire salvage of Picea mariana and Pinus banksiana. Forest Ecology and Management 303: 20–24. https://doi.org/10.1016/j.foreco.2013.03.049.
Hébert F., Boucher J.F., Walsh D., Tremblay P., Côté D., Lord D. (2014). Black spruce growth and survival in boreal open woodlands 10 years following mechanical site preparation and planting. Forestry 87(2): 277–286. https://doi.org/10.1093/forestry/cpt052.
Hogg E.H. (1994). Climate and the southern limit of the western Canadian boreal forest. Canadian Journal of Forest Research 24(9): 1835–1845. https://doi.org/10.1139/x94-237.
Hogg E.H., Hurdle P.A. (1995). The aspen parkland in western Canada: a dry-climate analogue for the future boreal forest? In: Boreal Forests and Global Change. Springer Netherlands. p. 391–400. https://doi.org/10.1007/978-94-017-0942-2_37.
Houle G., Filion L. (2003). The effects of lichens on white spruce seedling establishment and juvenile growth in a spruce-lichen woodland of subarctic Quebec. Ecoscience 10(1): 80–84. https://doi.org/10.1080/11956860.2003.11682754.
Hugron S., Andersen R., Poulin M., Rochefort L. (2011). Natural plant colonization of borrow pits in boreal forest highlands of eastern Canada. Botany 89(7): 451–465. https://doi.org/10.1139/b11-036.
Hustich I. (1951). The lichen woodlands in Labrador and their importance as winter pastures for domesticated reindeer. Acta Geographica 12: 1–48.
Jasinski J., Payette S. (2005). The creation of alternative stable states in the southern boreal forest, Quebec, Canada. Ecological Monographs 75(4): 561–583. https://doi.org/10.1890/04-1621.
Johnson E.A., Miyanishi K. (1999). Subarctic lichen woodlands. In: Anderson R., Fralish J., Baskin J. (eds.). Savanna, barren and rock outcrop plant communities of North America. Cambridge University Press, Cambridge. p. 421–436. https://doi.org/10.1017/CBO9780511574627.027.
Johnstone J.F., Hollingsworth T.N., Chapin F.S., Mack M.C. (2010). Changes in fire regime break the legacy lock on successional trajectories in Alaskan boreal forest. Global Change Biology 16(4): 1281–1295. https://doi.org/10.1111/j.1365-2486.2009.02051.x.
Kershaw K.A. (1977). Studies on the lichen-dominated systems. XX. An examination of some aspects of the northern boreal lichen woodlands in Canada. Canadian Journal of Botany 55(4): 393–410. https://doi.org/10.1139/b77-050.
Kershaw K.A. (1978). The role of lichens in boreal tundra transition areas. Bryologist 81(2): 294–306. https://doi.org/10.2307/3242190.
Lafleur B., Zouaoui S., Fenton N.J., Drapeau P., Bergeron Y. (2016). Short-term response of Cladonia lichen communities to logging and fire in boreal forests. Forest Ecology and Management 372: 44–52. https://doi.org/10.1016/j.foreco.2016.04.007.
Lambert J.D.H., Maycock P.F. (1968). The ecology of terricolous lichens of the Northern Conifer – hardwood forests of central Eastern Canada. Canadian Journal of Botany 46(8): 1043–1078. https://doi.org/10.1139/b68-138.
Maikawa E., Kershaw K.A. (1976). Studies on lichen-dominated systems. XIX. The postfire recovery sequence of black spruce–lichen woodland in the Abitau Lake Region, NWT. Canadian Journal of Botany 54(23): 2679–2687. https://doi.org/10.1139/b76-288.
McMullin T., Anderson F. (2014). Common lichens of northeastern North America. New York Botanical Garden Press, USA. 183 p.
Miyanishi K., Johnson E.A. (2002). Process and patterns of duff consumption in the mixedwood boreal forest. Canadian Journal of Forest Research 32(7): 1285–1295. https://doi.org/10.1139/x02-051.
Morneau C., Payette S. (1989). Postfire lichen-spruce woodland recovery at the limit of the boreal forest in northern Quebec. Canadian Journal of Botany 67(9): 2770–2782. https://doi.org/10.1139/b89-357.
Moss M., Hermanutz L. (2009). Postfire seedling recruitment at the southern limit of lichen woodland. Canadian Journal of Forest Research 39(12): 2299–2306. https://doi.org/10.1139/X09-150.
Oksanen J., Ahti T. (1982). Lichen-rich pine forest vegetation in Finland. Annales Botanici Fennici 19: 275–301.
Pacé M. (2017). Rôle de la strate des mousses et lichens dans l’établissement et le maintien de milieux ouverts stables en forêt boréale. [The role of moss and lichen stratum in the establishment and maintenance of stable open environments in the boreal forest]. Thèse de doctorat en sciences de l’environnement, Université du Québec en Abitibi-Témiscamingue, Rouyn-Noranda (QC). 253 p.
Payette S., Delwaide A. (2003). Shift of boreal forest to lichen: heath parkland caused by successive stand disturbances. Ecosystems 6(6): 540–550. https://doi.org/10.1007/PL00021507.
Payette S., Bhiry N., Delwaide A., Simard M. (2000). Origin of the lichen woodland at its southern range limit in eastern Canada: the catastrophic impact of insect defoliators and fire on the spruce-moss forest. Canadian Journal of Forest Research 30(2): 288–305. https://doi.org/10.1139/x99-207.
Peng C., Ma Z., Lei X., Zhu Q., Chen H., Wang W., Liu S., Li W., Fang X., Zhou X. (2011). A drought-induced pervasive increase in tree mortality across Canada’s boreal forests. Nature Climate Change 1(9): 467–471. https://doi.org/10.1038/nclimate1293.
Pinno B.D., Errington R.C., Thompson D.K. (2013). Young jack pine and high severity fire combine to create potentially expansive areas of understocked forest. Forest Ecology and Management 310: 517–522. https://doi.org/10.1016/j.foreco.2013.08.055.
Purvis O.W., Coppins B.J., Hawksworth D.L., James P.W., Moore D.M. (eds.). (1992). The lichen flora of Great Britain and Ireland. Natural History Museum Publications, London U.K.
Ramault J.L., Corvisier M. (1975). Inhibitory effect of Cladonia impexa Harm., C. gracilis (L.) Willd and Cornicularia muricata (Ach.) Ach. on Pinus sylvestris seed germination. Oecologia Plantarum 10: 295–299.
Rapanoela R., Raulier F., Gauthier S., Ouzennou H., Saucier J.-P., Bergeron Y. (2015). Contrasting current and potential productivity and the influence of fire and species composition in the boreal forest: a case study in eastern Canada. Canadian Journal of Forest Research 45(5): 541–552. https://doi.org/10.1139/cjfr-2014-0124.
Raulier F., Le Goff H., Gauthier S., Rapanoela R., Bergeron Y. (2013). Introducing two indicators for fire risk consideration in the management of boreal forests. Ecological Indicators 24: 451–461. https://doi.org/10.1016/j.ecolind.2012.07.023.
Roturier S., Bäcklund S., Sundén M., Bergsten U. (2007). Influence of ground substrate on establishment of reindeer lichen after artificial dispersal. Silva Fennica 41(2): 269–280. https://doi.org/10.14214/sf.296.
Rouse W.R., Kershaw K.A. (1971). The effects of burning on the heat and water regimes of lichen-dominated subarctic surfaces. Arctic and Alpine Research 3(4): 291–304. https://doi.org/10.2307/1550045.
Rowe J.S. (1972). Forest regions of Canada. Forest Service Publication NO. 1300.
Rudolph T.D., Laidly P.R. (1990). Pinus banksiana (Lamb.) Jack pine. In: Burn R.M., Honkala B.H. (eds.). Silvics of North America: 1. conifers. Agriculture handbook 654. USDA Forest Service, Washington, DC. p. 280–293.
Sedia E.G., Ehrenfeld J.G. (2003). Lichens and mosses promote alternate stable plant communities in the New Jersey pinelands. Oikos 100(3): 447–458. https://doi.org/10.1034/j.1600-0706.2003.12058.x.
Sirois L. (1993). Impact of fire on Picea mariana and Pinus banksiana seedlings in subarctic lichen woodlands. Journal of Vegetation Science 4(6): 795–802. https://doi.org/10.2307/3235617.
Splawinski T.B., Greene D.F., Gauthier S. (2014). A model of the post-fire recruitment of Picea mariana and Pinus banksiana as a function of salvage timing and intensity. Ecological Modeling 282: 35–43. https://doi.org/10.1016/j.ecolmodel.2014.03.007.
Splawinski T.B., Gauthier S., Bergeron Y., Greene D.F. (Unpublished). Juvenile stage regeneration dynamics of Pinus banksiana and Picea mariana following fire, salvage, and silvicultural treatment.
Terrier A., de Groot W.J., Girardin M.P., Bergeron Y. (2014). Dynamics of moisture content in spruce–feather moss and spruce–Sphagnum organic layers during an extreme fire season and implications for future depths of burn in Clay Belt black spruce forests. International journal of wildland fire 23(4): 490–502. https://doi.org/10.1071/WF13133.
Terrier A., Girardin M.P., Cantin A., Groot W.J., Anyomi K.A., Gauthier S., Bergeron Y. (2015). Disturbance legacies and paludification mediate the ecological impact of an intensifying wildfire regime in the Clay Belt boreal forest of eastern North America. Journal of Vegetation Science 26(3): 588–602. https://doi.org/10.1111/jvs.12250.
Thiffault N., Roy V., Prégent G., Cyr G., Jobidon R., Ménétrier J. (2003). La sylviculture des plantations résineuses. [The silviculture of conifer plantations]. Le Naturaliste Canadien 127: 63–80.
Thiffault N., Roy V., Ménétrier J., Prégent G., Rainville A. (2013). La plantation. [Plantations]. In: Larouche C., Guillemette F., Raymond P., Saucier J.-P. (eds.). Le guide sylvicole du Québec. Tome 2. Les concepts et l’application de la sylviculture. Québec, QC. p. 196–225.
Tremblay P., Boucher J.F., Tremblay M., Lord D. (2013). Afforestation of boreal open woodlands: early performance and ecophysiology of planted black spruce seedlings. Forests 4(2): 433–454. https://doi.org/10.3390/f4020433.
Van Bogaert R., Gauthier S., Drobyshev I., Jayen K., Greene D.F., Bergeron Y. (2015a). Prolonged absence of disturbance associated with increased environmental stress may lead to reduced seedbank size in Picea mariana in boreal eastern North America. Ecosystems 18(7): 1135–1150. https://doi.org/10.1007/s10021-015-9888-3.
Van Bogaert R., Gauthier S., Raulier F., Saucier J.P., Boucher D., Robitaille A., Bergeron Y. (2015b). Exploring forest productivity at an early age after fire: a case study at the northern limit of commercial forests in Quebec. Canadian Journal of Forest Research 45(5): 579–593. https://doi.org/10.1139/cjfr-2014-0273.
Viereck L.A., Foote M.J. (1979). Effect of burning on soil temperature. In: Viereck L.A., Dyrness C.T. (eds.). Ecological effects of the Wickersham Dome fire near Fairbanks, Alaska. USDA Forest Service General Technical Report PNW-90. p. 14–17.
Viereck L.A., Johnston W.F. (1990). Picea mariana (Mill.) B.S.P. black spruce. In: Burn R.M., Honkala B.H. (eds.). Silvics of North America: 1. conifers. Agriculture handbook 654. USDA Forest Service, Washington, DC. p. 227–237.
Wotton B.M., Flannigan M.D. (1993). Length of the fire season in a changing climate. Forestry Chronicle 69(2): 187–192. https://doi.org/10.5558/tfc69187-2.
Wotton B.M., Nock C.A., Flannigan M.D. (2010). Forest fire occurrence and climate change in Canada. International Journal of Wildland Fire 19(3): 253–271. https://doi.org/10.1071/WF09002.
Total of 90 references.

