Infection of pre-commercially cut stumps of Picea abies and Pinus sylvestris by Heterobasidion spp. – a comparative study
Gaitnieks T., Brauners I., Kenigsvalde K., Zaļuma A., Brūna L., Jansons J., Burņeviča N., Lazdiņš A., Vasaitis R. (2018). Infection of pre-commercially cut stumps of Picea abies and Pinus sylvestris by Heterobasidion spp. – a comparative study. Silva Fennica vol. 52 no. 1 article id 9911. https://doi.org/10.14214/sf.9911
Highlights
- In pre-commercial thinnings both Heterobasidion infection frequency and the extent of surface colonization correlated positively with stump diameter of both Norway spruce and Scots pine
- Spruce stumps were significantly more often subjected to primary infections than pine stumps
- The pathogen exhibited more extensive surface colonization of spruce stumps than of pine stumps.
Abstract
The aim was to investigate relative susceptibility of stumps of spruce and pine to airborne infections by Heterobasidion following pre-commercial thinnings. The proportions of infected stumps and colonized stump surface areas were analysed in 16 forest stands. In total, 746 spruce and 1063 pine stumps were sampled, and 184 and 105 infected stumps, respectively, were analysed. In conclusion, the present study demonstrated that in the investigated area: i) both Heterobasidion infection frequency and the extent of surface colonization correlated positively with stump diameter of both spruce and pine; ii) spruce stumps were significantly more often subjected to primary infections than pine stumps; iii) the pathogen exhibited more extensive surface colonization of spruce stumps than of pine stumps.
Keywords
Norway spruce;
Scots pine;
pre-commercial thinnings;
Heterobasidion
-
Gaitnieks,
Latvian State Forest Research Institute “Silava”, 111 Rigas str., Salaspils, LV-2169, Latvia
E-mail
talis.gaitnieks@silava.lv

- Brauners, JSC “Latvia’s State Forests”, 1 Vainodes str., Riga, LV-1004, Latvia E-mail i.brauners@lvm.lv
- Kenigsvalde, Latvian State Forest Research Institute “Silava”, 111 Rigas str., Salaspils, LV-2169, Latvia E-mail kristine.kenigsvalde@silava.lv
- Zaļuma, Latvian State Forest Research Institute “Silava”, 111 Rigas str., Salaspils, LV-2169, Latvia E-mail astra.zaluma@silava.lv
- Brūna, Latvian State Forest Research Institute “Silava”, 111 Rigas str., Salaspils, LV-2169, Latvia E-mail lauma.bruna@silava.lv
- Jansons, Latvian State Forest Research Institute “Silava”, 111 Rigas str., Salaspils, LV-2169, Latvia E-mail jurgis.jansons@silava.lv
- Burņeviča, Latvian State Forest Research Institute “Silava”, 111 Rigas str., Salaspils, LV-2169, Latvia E-mail natalija.arhipova@silava.lv
- Lazdiņš, Latvian State Forest Research Institute “Silava”, 111 Rigas str., Salaspils, LV-2169, Latvia E-mail andis.lazdins@silava.lv
- Vasaitis, Swedish University of Agricultural Sciences (SLU), P.O. Box 7026, SE-75007 Uppsala, Sweden E-mail Rimvys.Vasaitis@slu.se
Received 17 November 2017 Accepted 23 January 2018 Published 24 January 2018
Views 60426
Available at https://doi.org/10.14214/sf.9911 | Download PDF

1 Introduction
Species of Heterobasidion (H. parviporum Niemelä and Korhonen and H. annosum (Fr.) Bref.) in forests of northern Hemisphere cause serious economic losses to Norway spruce (Picea abies (L.) H. Karst.) and Scots pine (Pinus sylvestris L.) (Korhonen and Holdenrieder 2005). Airborne basidiospores of Heterobasidion colonize freshly cut stumps, and from infected stumps these fungi spread along tree roots to healthy adjacent trees (Woodward et al. 1998). Stumps left after pre-commercial thinning generally have not been considered important for infection (Vollbrecht et al. 1995; Bendz-Hellgren and Stenlid 1998), although some investigations demonstrated that such stumps, e.g. of spruce, are to certain extent susceptible (Paludan 1966; Berglund et al. 2008). For example, 55% of pre-commercially cut stumps of spruce (298 examined, diameter 2.4–14.5 cm, tree age 8–15 years) were found to be infected by airborne spores of Heterobasidion in southern Sweden (Gunulf et al. 2012).
Moreover, more recent study has demonstrated the ability of Heterobasidion to spread from artificially inoculated 2–14 cm spruce stumps to adjacent trees, indicating their silvicultural importance. Moreover, simulation model has demonstrated that predicted probability of airborne infections is likely to increase with increasing stump diameter (Gunulf et al. 2013).
Available investigations on Heterobasidion infection to small-diameter stumps of Scots pine are also scarce. In East England 2 out of 30 (6.7%) investigated stumps (diameter 5–7 cm) were naturally infected by airborne Heterobasidion spores three years after cutting, while following artificial inoculations to four-year-old stumps, established infections were observed in 20 out of 50 (40%) after 7 months (Rishbeth 1951). In Finland, airborne infection by Heterobasidion was detected in 47 out of 1375 (3.4%) examined stumps in thinned 10–16 year-old pine stands during two months – one year since cutting (Jokinen 1984).
Therefore, in order to get more insights into infection biology of Heterobasidion in young plantations of spruce and pine, more investigations are required, in particularly directed towards relative susceptibility of those tree species to primary infections of the disease and subsequent establishment of the pathogen in a stand. Also, more knowledge is needed to answer the question, how those processes are influenced by stump diameter / tree age. The results might potentially have certain silvicultural implications, e.g. which species to prefer for planting in certain areas, what density, at which diameter first thinnings should be carried out, and whether stumps should be treated against Heterobasidion.
So far, in this respect only a single study is available for stumps of spruce (Gunulf et al. 2013), but none for pine. The aim of the study was to investigate relative susceptibility of stumps of spruce and pine to airborne infections by Heterobasidion following pre-commercial thinnings in relation to diameter of cut stumps, stump age and tree age. To achieve this, the proportions of infected stumps and colonized stump surface areas were measured and analysed in a number of stands of both tree species.
2 Materials and methods
Experimental plots were established during June–September in 2006–2013 in seven plantations of Norway spruce, and nine plantations of Scots pine established on former forest land located in eastern Latvia (52°42´N, 25°50´E) within a radius of 10 km. Previous forest generation consisted either of birch (in 4 plots of spruce and 2 pine) or pine (in 2 of spruce and 7 pine) (information for one plot unavailable). Age of the stands was 10–39 years (Table 1), and plot area 0.7–3.5 ha. In each plot, 53–194 trees were cut using a chainsaw. In total, 746 and 1063 trees were cut, respectively of spruce and pine (Table 1), during June–September. Height of stumps was 20–50 cm, diameter 1.5–12.1 cm (Table 1), and none of them showed decay symptoms.
| Table 1. Studied stands, stumps and Heterobasidion infections. | |||
| Norway spruce | Scots pine | Significance a at α = 0.05 | |
| Stands and stumps | |||
| Stand age, years (min – max) | 11 – 39 | 10 – 15 | - |
| – mean ± SD | 24.4 ± 10.4 | 12.8 ± 1.4 | p = 0.04 |
| Number of investigated stumps | 746 | 1063 | - |
| Stump age at sampling, weeks (min – max) | 7 – 260 | 9 – 260 | - |
| – mean ± SD | 68.4 ± 91.6 | 67.8 ± 77.4 | p = 0.52 |
| Stump diameter, cm (min – max) | 2.1 – 12.1 | 1.5 – 11.7 | - |
| – mean ± SD | 6.1 ± 2.1 | 5.1 ± 1.8 | p > 0.05 |
| Heterobasidion infections | |||
| Number of infected stumps | 184 | 105 | - |
| Infection frequency, % of infected stumps (min – max) | 1.3 – 84.9 | 0.0 – 36.9 | - |
| – mean ± SD | 25 ± 3 | 10 ± 2 | p < 0.05 |
| Colonized stump surface area, cm2 (min – max) | 0.5 – 55.4 | 0.5 – 53.9 | - |
| – mean ± SD | 7.5 ± 8.4 | 4.1 ± 6.3 | p < 0.05 |
| Colonized stump surface area, % (min – max) | 0.4 – 68.9 | 0.8 – 81.1 | - |
| – mean ± SD | 21.7 ± 17.5 | 11.0 ± 11.5 | p < 0.05 |
| a difference between tree species | |||
The stumps were sampled ca. 7–260 weeks after cutting (Table 1). Two 3–4 cm thick discs were cut from each stump with a chainsaw. The first (top) disc was discarded and the second disc was taken to laboratory. There the discs were debarked, washed with a stiff brush under running tap water and placed in loosely closed plastic bags. After incubation for 5–7 days at room temperature a grid consisting of 0.5 cm2 squares was fixed on to the lower side of the disc (stump cross section). Discs were examined for the presence of Heterobasidion conidiophores, using a dissection microscope at 20–30 times magnification. Grid squares where conidiophores were found were marked on the disc. The number of marked squares was counted, and the surface area (cm2) of wood colonized by Heterobasidion on stump cross-sections was estimated (Kenigsvalde et al. 2016).
Among all examined stumps, 184 (25%) spruce and 105 (10%) pine stumps were Heterobasidion-infected, and subsequently included into the further analyses (Table 1). Infection frequencies (number of infected stumps) between tree species were compared using a chi-square test. Interspecific comparison of cross-section surface areas colonized by Heterobasidion in spruce and pine was accomplished using non-parametric Mann-Whitney test. Correlations between stump diameter and infection frequency, stump surface colonized by Heterobasidion, and stump age (time since cutting) were calculated. Tests were performed in RStudio (R Core Team 2015).
3 Results
Examined stands of spruce were older than those of pine, and the difference was statistically significant (Table 1). However, there were no interspecific differences neither between the age of sampled stumps, nor between their diameter. As our sample plots were located within a radius of ca. 10 km, this provides certain background for comparison of both frequencies of Heterobasidion infection and the extent of surface colonization in stumps of spruce and pine. Pooled analysis of Heterobasidion infection frequencies detected on former birch (703 stumps) and pine (1018 stumps) sites demonstrated that, respectively, 97 (13.8%) and 118 (11.6%) of those were infected, thus more often on birch than on pine sites, although the difference was non-significant (chi-squared test, p = 0.17). Despite compact geographic area, the infection frequency varied greatly between stands, ranging in the seven spruce stands from 1.3 to 84.9% (mean 25%), and in nine pine stands from 0to 36.9% (10%), and the difference was statistically significant (Table 1). Significantly larger proportion of spruce stumps were infected as compared with pine stumps (Table 1). In both tree species, infection frequencies were significantly higher in stumps of larger diameter, more evidently in pine than in spruce (Fig. 1). In spruce, higher incidence of infection was observed in younger stumps (p < 0.001), while no relationship was found between infection frequency and stump age in pine (p = 0.23). Stump age did not have significant effect on surface colonization area neither in spruce (r = 0.08, p = 0.27), nor in pine (r = 0.18, p = 0.06). There was no correlation between colonized stump surface area and tree age neither in stumps of spruce (r = 0.09, p = 0.23) nor pine (r = 0.01, p = 0.92). The area varied between 0.5–55.4 cm2 (mean 7.5 cm2) in spruce and between 0.5–53.9 cm2 (4.1 cm2) in pine, and the difference was statistically significant (Table 1). There was a clear statistically significant trend to observe larger Heterobasidion colonies in stumps of larger diameter for both tree species, and this trend was more sharply pronounced in spruce (Fig. 2). The proportion of colonized stump surface area varied between 0.4–68.9% (mean 21.7%) in spruce stumps, and between 0.8–81.1% (11.0%) in pine stumps, the difference being statistically significant (Table 1).
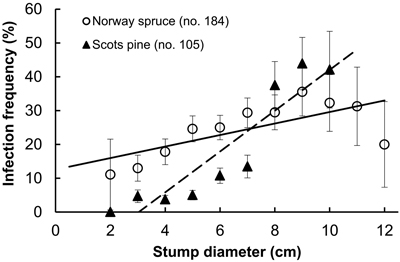
Fig. 1. Infection frequency by Heterobasidion to stumps of Norway spruce and Scots pine in relation to stump diameter; spruce: r = 0.71, (p = 0.02), y = 1.7x + 12.6; pine: r = 0.97 (p < 0.001), y = 6.0x–18.3 (dashed). Bars show standard error.
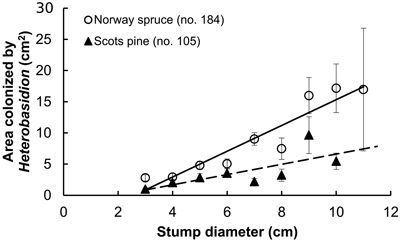
Fig. 2. Stump surface area colonized by Heterobasidion in relation to stump diameter; spruce: r = 0.46 (p < 0.001), y = 2.1x–5.4; pine: r = 0.33 (p < 0.001), y = 0.8x–1.6 (dashed). Bars show standard error.
4 Discussion
Since there were no records of dieback in previous generation stands and there was no difference in Heterobasidion incidence on former birch (previous generation non-susceptible to spore infections) and pine sites (potentially susceptible), presented data presumably reflects prevailing primary (airborne) infections. The results support findings of few available related studies. Similarly, Rönnberg et al. (2006) reported infection frequency of Heterobasidion to pine stumps in adjacent stands to be highly variable, 3.3–46.7%. Such high variation could probably be explained by eventually scattered distribution of Heterobasidion sporocarps in investigated areas (as typically this is the case). It is known, that although airborne spores of the fungus have potential to spread over 100s of kilometres, prevailing majority of them land in the vicinity of a sporocarp (Rishbeth 1951; Stenlid 1994).
That spruce stumps are more prone to Heterobasidion than pine has been previously observed in another Latvian study (Kenigsvalde et al. 2016), where in mixed stands primary infections by Heterobasidion were observed in 81–85% of spruce stumps, compared with 0–14% of pine. More frequent infections to larger stumps have also been previously reported both in spruce (Paludan 1966; Solheim 1994; Bendz-Hellgren and Stenlid 1998) and pine (Rishbeth 1951). Colonized surface area detected in this work is also comparable with the limited data available so far: Gunulf et al. (2012) found average area of 3.5 cm2 in 10 examined pre-commercially cut spruce stumps after 2 months since cutting, mean proportion constituting 11.7% of the surface. High variability in area of colonized stump wood has been noted even in artificially basidiospore-inoculated stumps, as e.g. of Sitka spruce (0.02–56.6%) (Morrison and Redfern 1994) and lodgepole pine (3–96%) (Redfern 1982).
In conclusion, the present study demonstrated that in the investigated area in pre-commercially cut stumps: i) both Heterobasidion infection frequency and the extent of surface colonization correlated positively with stump diameter of both spruce and pine; ii) spruce stumps were significantly more often subjected to primary infections than pine stumps; iii) the pathogen exhibited more extensive surface colonization of spruce stumps than of pine stumps.
Acknowledgements
This work was supported by the JSC “Latvia’s State Forests” and State research programme “Forest and earth entrails resources: research and sustainable utilization – new products and technologies” (ResProd) project “Even-age spruce stands cultivation potential in fertile forest ecosystem”. Research was carried out in accordance with the contract No. 1.2.1.1/16/A/009 between “Forest Sector Competence Centre” Ltd. and the Central Finance and Contracting Agency, concluded on 13th of October, 2016, the study is conducted by the Latvian State Forest Research Institute “Silava” with support from the European Regional Development Fund (ERDF) within the framework of the project “Forest Sector Competence Centre”.
References
Bendz-Hellgren M., Stenlid J. (1998). Effects of clear-cutting, thinning, and wood moisture content on the susceptibility of Norway spruce stumps to Heterobasidion annosum. Canadian Journal of Forest Research 28(5): 759–765. http://dx.doi.org/10.1139/x98-043.
Berglund M., Carlsson T., Rönnberg J. (2008). Infection of Heterobasidion spp. in late pre-commercial thinnings of Picea abies in southern Sweden. In: Garbelotto M., Gonthier P., (eds.). Proceedings of the 12th International Conference on Root and Butt Rots of Forest Trees, 12–19 August 2007, Berkeley, California, University of California. p. 221–225. ISBN 9780615230764.
Gunulf A., Mc Carthy R., Rönnberg J. (2012). Control efficacy of stump treatment and influence of stump height on natural spore infection by Heterobasidion spp. of precommercial thinning stumps of Norway spruce and birch. Silva Fennica 46(5): 655–665. http://dx.doi.org/10.14214/sf.917.
Gunulf A., Wang L., Englund J.E., Rönnberg J. (2013). Secondary spread of Heterobasidion parviporum from small Norway spruce stumps to adjacent trees. Forest Ecology and Management 287: 1–8. http://dx.doi.org/10.1016/j.foreco.2012.09.011.
Jokinen K. (1984). Männyn tyvitervastaudin leviäminen ja torjunta harmaaorvakalla (Phlebiopsis gigantea) männyn taimikoiden harvennuksessa. [The spread of Heterobasidion annosum and its control using Phlebiopsis gigantea during thinnings in the young stands of Scots pine]. Folia Forestalia 607: 1–12. http://urn.fi/URN:ISBN:951-40-0681-X. [In Finnish].
Kenigsvalde K., Brauners I., Korhonen K., Zaļuma A., Mihailova A., Gaitnieks T. (2016). Evaluation of the biological control agent Rotstop in controlling the infection of spruce and pine stumps by Heterobasidion in Latvia. Scandinavian Journal of Forest Research 31(3): 254–261. http://dx.doi.org/10.1080/02827581.2015.1085081.
Korhonen K., Holdenrieder O. (2005). Neue Erkenntnisse über den Wurzelschwamm (Heterobasidion annosum s.l.). Eine Literaturübersicht. [Recent advances in research on the root rot fungus Heterobasidion annosum s.l. A literature review]. Forst und Holz 60(5): 206–211. [In German].
Morrison D.J., Redfern D.B. (1994). Long-term development of Heterobasidion annosum in basidiospore-infected Sitka spruce stumps. Plant Pathology 43(5): 897–906. http://dx.doi.org/10.1111/j.1365-3059.1994.tb01634.x.
Paludan F. (1966). Infektion og spredning af Fomes annosus i ung Rødgran. [Infection and spread of Fomes annosus in young Norway Spruce]. Det forstlige førsøksvæsen i Danmark 30: 19–47. [In Danish].
R Core Team. (2015). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Redfern D.B. (1982). Infection of Picea sitchensis and Pinus contorta stumps by basidiospores of Heterobasidion annosum. European Journal of Forest Pathology 12(1): 11–25. http://dx.doi.org/10.1111/j.1439-0329.1982.tb01367.x.
Rishbeth J. (1951). Observations on the biology of Fomes annosus, with particular reference to East Anglian pine plantations. III Natural and experimental infection of pines, and some factors affecting severity of the disease. Annals of Botany 15(2): 221–246. http://dx.doi.org/10.1093/oxfordjournals.aob.a083278.
Rönnberg J., Petrylaite E., Nilsson G., Pratt J. (2006). Two studies to assess the risk to Pinus sylvestris from Heterobasidion spp. in southern Sweden. Scandinavian Journal of Forest Research 21(5): 405–413. http://dx.doi.org/10.1080/02827580600917379.
Solheim H. (1994). Infeksjon av rotkjuke på granstubber til ulike årstider og effekten av ureabehandling. [Seasonal infection of Heterobasidion annosum on stumps of Norway spruce and surface coating with urea]. Norsk Institutt for Skogforskning, Rapport fra Skogforsk 3/94. 10 p. [In Norwegian].
Stenlid J. (1994). Regional differentiation in Heterobasidion annosum. In: Johansson M., Stenlid J. (eds.). Proceedings of Eighth International Conference on Root and Butt Rots; 9–16 August 1993, Wik, Sweden, Haikko, Finland. Swedish University of Agricultural Sciences. p. 243–248.
Vollbrecht G., Gemmel P., Pettersson N. (1995). The effect of precommercial thinning on the incidence of Heterobasidion annosum in planted Picea abies. Scandinavian Journal of Forest Research 10(1–4): 37–41. http://dx.doi.org/10.1080/02827589509382865.
Woodward S., Stenlid J., Karjalainen R., Hüttermann A. (1998). Heterobasidion annosum: biology, ecology, impact and control. CAB International, Wallingford. 589 p. ISBN 0851992757.
Total of 17 references.

