Effects of pre-harvest fertilization and subsequent soil scarification on the growth of planted Pinus sylvestris seedlings and ground vegetation after clear-felling
Johansson K., Ring E., Högbom L. (2013). Effects of pre-harvest fertilization and subsequent soil scarification on the growth of planted Pinus sylvestris seedlings and ground vegetation after clear-felling. Silva Fennica vol. 47 no. 4 article id 1016. https://doi.org/10.14214/sf.1016
Highlights
- Pre-harvest N fertilization had no significant effect on seedling growth and ground vegetation biomass
- Scarification improved seedling survival and growth and reduced the amount of ground vegetation
- Without scarification, pre-harvest fertilization increased the amount of damaged seedlings.
Abstract
Fertilization and scarification are both performed to increase tree production at different stages of forest rotation periods. In this study, the effects of previous nitrogen fertilizations and scarification after clear felling on planted Pinus sylvestris L. seedlings and ground vegetation were investigated. Two fertilization experiments established around 1980 were harvested in 2006, after which the plots were scarified by disc trenching and re-planted. The plots had been repeatedly fertilized over a 20-year period before harvesting, with total N doses of 0, 450, 900 or 1800 kg N ha-1. After five growing seasons, the growth, survival and nutrient contents of the seedlings were measured, and ground vegetation was collected to estimate its biomass and nutrient content. Pre-harvest fertilization alone had only minor effects on the results, but scarification increased both the survival and growth of the planted seedlings. However, without scarification, seedling mortality increased with increasing fertilization intensity. The ground vegetation biomass was higher in plots without scarification, but the total biomass of seedlings and ground vegetation was similar in all treatments. Scarification thus favored seedling growth at the expense of ground vegetation. Only a few effects on nutrient content were found, but there were no signs of nutrient imbalance in any of the treatments. At higher levels of fertilization, the K:N ratio in the seedlings decreased while the K content in the ground vegetation increased. Overall, scarification had a greater impact than pre-harvest fertilization on the planted seedlings and the ground vegetation.
Keywords
nitrogen;
establishment;
regeneration;
Scots pine;
disc trenching;
carryover;
competing vegetation
-
Johansson,
Skogforsk, The Forestry Research Institute of Sweden, Ekebo 2250, SE-268 90 Svalöv, Sweden & Southern Swedish Forest Research Centre, SLU, Box 49, SE-230 53 Alnarp, Sweden
E-mail
karin.johansson@skogforsk.se

- Ring, Skogforsk, The Forestry Research Institute of Sweden, Uppsala Science Park, SE-751 83 Uppsala, Sweden E-mail eva.ring@skogforsk.se
- Högbom, Skogforsk, The Forestry Research Institute of Sweden, Uppsala Science Park, SE-751 83 Uppsala, Sweden E-mail lars.hogbom@skogforsk.se
Received 11 April 2013 Accepted 22 August 2013 Published 17 October 2013
Views 165693
Available at https://doi.org/10.14214/sf.1016 | Download PDF

1 Introduction
Forests provide many ecosystem services: they produce raw materials that are used by the timber, pulp, paper, and bioenergy industries and are also important in the preservation of biodiversity, the maintenance of good water quality, and as locations for recreational activity. To be able to fulfil the demands from forests, there is a growing desire to increase the productive output (Regeringens proposition 2008), which could potentially be achieved by reducing the rotation periods from regeneration to final cutting. Two widely used measures for promoting tree growth are fertilization of mature stands and soil scarification following final felling.
The availability of nitrogen (N) is one of the major growth-limiting factors in many boreal forests growing on mineral soil (Tamm 1991; Bergh et al. 1999). By adding N to suitable middle-aged or older stands, stem-wood growth can be significantly increased (Pettersson 1994; Jacobson and Pettersson 2010). However, N fertilization affects a number of other parts of the ecosystem in addition to promoting tree growth. For example, the composition of the forest floor vegetation usually changes rapidly following fertilization, due to both the increased availability of N and the decrease in light penetration through the denser tree canopy (Hedwall et al. 2010). The environmental effects of N fertilization have been examined in numerous studies. In most cases, these investigations focused primarily on effects occurring during the current rotation period. However, N fertilization may have effects that persist into the next rotation period. Large pre-harvest fertilizer doses can have negative effects on soil-solution chemistry after final felling (Ring 2004), although results that contradict this conclusion have also been presented (Ring et al. 2013). Strengbom and Nordin (2008) reported strong effects on ground vegetation composition after the felling and regeneration of commercially fertilized forests. Other studies have shown that fertilization with practically relevant N dosages have only minute effects on ground vegetation (Olsson and Kellner 2006).
Scarification promotes the growth of planted seedlings by alternating their microenvironments, i.e. the availability or abundance of site resources such as nutrients and soil moisture as well as the soil temperature (Brand 1991; Grossnickle 2000; Johansson et al. 2007). An important aspect of scarification is the removal of competing vegetation, which will give the seedlings an initial growth advantage over both woody and herbaceous species colonizing the clear felled area (Prevost 1992; Munson et al. 1993; Thiffault et al. 2005). It has also been found that the positive effects of scarification persist over time, i.e. the initial growth gains are maintained in the long run and a more homogeneous stand is developed compared to stands established without the use of scarification (Nilsson and Allen 2003; Johansson et al. 2013). Although scarification is mainly performed to promote seedling establishment and growth, it also protects the seedlings from being attacked by pine weevils (Hylobius abietis) (Petersson et al. 2005; Nordlander et al. 2011). Damage caused by pine weevils is a common problem when regenerating conifers in large parts of Europe (Day and Leather 1997). The importance of scarification as a measure to reduce pine weevil damage will probably increase in the future, primarily due to the prohibition of insecticides that are currently used to protect against pine weevil attacks.
Despite extensive research on fertilization and scarification, the effects of pre-harvest fertilization and its interactions with post-harvest scarification on seedling growth remain poorly understood. Two previous studies did not reveal any effect of pre-harvest fertilization on the growth and survival of conifer seedlings, but the seedlings in question were planted in undisturbed or very mildly disturbed soil (Högbom et al. 2001; Sikström 2005). On the contrary, an increased seedling growth has been found as a carryover effect of N fertilization in previous stands on low quality sites in the Pacific Northwest (Footen et al. 2009). Since both pre-harvest fertilization and scarification alone affects the growth environment, it is important to identify any interactions that may appear between them. Furthermore, since the main part of the N applied at fertilization is typically incorporated into the soil (Melin and Nömmik 1988; Nohrstedt 1990; Nohrstedt 2001), effects of pre-harvest fertilization may develop after scarification as a result of increased mineralization.
The long-term effects of different N fertilization regimes have been studied in several field experiments. A series of such experiments were established in Sweden around 1980 (Jacobson and Pettersson 2010). As expected, stem-wood growth increased significantly with increasing N fertilization intensity, but the relative growth response was stronger under less intense fertilization regimes. Two of the sites in this experimental series were clear-felled, scarified and replanted in 2006. A study conducted one year before clear-felling showed that with increasing fertilization intensity, larger effects on the soil chemistry were found (Ring et al. 2011). The aim of the study reported herein was to determine whether or not pre-harvest fertilization and scarification affect the growth and nutrient status of the next generation of planted seedlings and the ground vegetation. Also, possible interaction effects between previous fertilization regime and scarification were to be investigated. We hypothesized that:
- Pre-harvest fertilization alone does not affect seedling growth, but in combination with scarification the growth rate is increased due to an increased amount of plant-available N in the soil.
- Scarification promotes seedling growth and survival by improving seedling establishment.
- The ratio of other nutrients (P, K, Ca, Mg and S) to N within the seedlings will decrease with increasing pre-harvest N fertilization intensity due to higher uptake of N.
- The total ground vegetation biomass will increase with the intensity of fertilization, but will be reduced by scarification.
2 Materials and methods
2.1 Experimental design
The experiments were established on podzolized and N-limited sites with a sandy-silty till soil at Hagfors (60°00´N, 13°42´E) and a sandy sediment at Nissafors (57°24´N, 13°37´E). The site quality index values in terms of volume growth were 5.9 m3 ha–1 year–1 at Hagfors and 5.5 m3 ha–1 year–1at Nissafors. For more details concerning the sites, see Ring et al. (2011, 2013). The original purpose of the Hagfors and Nissafors experiments was to investigate the effects of different levels of N-fertilization on stem-wood growth in middle-aged Scots pine (Pinus sylvestris L.) stands (Jacobson and Pettersson 2010). The design of the experiments conducted at Hagfors and Nissafors made it possible to study the effects of pre-harvest fertilization regimes on the regeneration of seedlings planted after clear-felling of the fertilized stands, as well as the associated effects on the colonization of the clear-cut area by ground vegetation. The fertilization treatments started in 1981 at Hagfors and in 1977 at Nissafors. A randomized block design was applied at both sites and each fertilization treatment was replicated three times in plots sized 30 m x 30 m. The study plots were located several meters apart from each other, but the distance varied. At Hagfors, four fertilization regimes were included in the current study, whereof one was the control, hereafter referred to as 0N. Starting in 1981, a dosage of 150 kg N ha–1 (NH4NO3) was applied at intervals of eight, four or two years to achieve total application levels of 450, 900 and 1800 kg N ha–1 (hereafter referred to as 450N, 900N and 1800N) over the course of the remaining rotation period (Table 1). At Nissafors, two fertilization treatments were considered, with total dosages of 0and 450 kg N ha–1 (Table 2).
| Table 1. N fertilizer application intervals for the N treatments at Hagfors. The dosage is shown as kg N ha–1. | ||||
| Year | Treatment | |||
| 0N | 450N | 900N | 1800N b) | |
| 1981 | - | 150 | 150 | 150 |
| 1983 | - | - | - | 150 |
| 1985 | - | - | 150 | 150 |
| 1987 | - | - | - | 150 |
| 1989 | - | 150 | 150 | 150 |
| 1991 a) | - | - | - | 150 |
| 1993 a) | - | - | 150 | 150 |
| 1995 a) | - | - | - | 150 |
| 1997 a) | - | 150 | 150 | 150 |
| 1999 a) | - | - | - | 150 |
| 2001 a) | - | - | 150 | 150 |
| 2003 a) | - | - | - | 150 |
| a) Dolomite was added to the fertilizer, which then contained 27% N, 5% Ca, 2% Mg and 0.2% B. b) At the first five applications, the three 1800N plots received different amounts of other nutrients (0 up to 10 kg per nutrient), see Ring et al. (2011) for more details. | ||||
| Table 2. N fertilizer application intervals for the different treatments at Nissafors. The dosage is shown as kg N ha–1. | ||
| Year | Treatment | |
| 0N | 450N | |
| 1977 | - | 75 (block 1 and 2), 6x25 (block 3) |
| 1978 | - | 75 (block 1 and 2) |
| 1984 | - | 150 |
| 1989 a) | - | 150 |
| a) In addition, 20 kg P ha–1 was applied. | ||
Both the Hagfors and the Nissafors stands were harvested in the spring of 2006, leaving no logging residues on the study plots. Each plot was split in two sub-plots (15 m x 30 m) of which one was disc trenched shortly after harvesting (scarification) while the other sub-plot was left undisturbed (no scarification). Disc trenching was performed in rows with 6–7 rows, two meters apart, in each sub-plot. Five rows per sub-plot were used in the present study, leaving the outermost rows as a buffer. During late May and early June of 2006, both sites were planted with 1.5 year old containerized Scots pine seedlings of suitable provenance. The seedlings were planted in rows with 2 m spacing in both disc trenched and undisturbed sub-plots, with 11 seedlings in each row. In the disc trenched sub-plots, seedlings were planted in the bare mineral soil in the middle of the furrows. On average, the furrows were 17 cm deep and 70 cm wide at Hagfors and 6 cm deep and 63 cm wide at Nissafors (Ring et al. 2013). A total of 1320 seedlings were planted and measured at Hagfors, and 660 at Nissafors. All seedlings were treated with insecticides (Merit Forest WG at Hagfors and Cyper Plus 2% at Nissafors) prior to planting and again during the following spring to prevent damage caused by pine weevil attacks. The seedlings at Nissafors were given an additional treatment in the spring of 2008. In contrast to Hagfors, the experimental site at Nissafors was fenced in order to protect against damage caused by large browsing animals.
2.2 Seedling and vegetation measurements
Seedling height (mm), root collar diameter (mm) and damage were assessed on the seedlings at the time of planting and five consecutive growing seasons after planting, in 2006–2010. In cases where a seedling had sustained damage, both the major cause of damage and its severity were recorded. The degree of damage was determined using a scale that ranged from 0–5, with a value of 0indicating no damage, 1 slightly damaged, 2 reduced growth, 3 strongly reduced growth, 4 expected to die, and 5 indicating that the seedling was dead.
In June 2011, five years after planting, needle samples for nutrient analysis were taken from ten randomly selected seedlings per sub-plot at Hagfors, a total of 240 samples. All needles were collected from shoots produced during the previous year (i.e. they were one year-old, C+1 needles). At the same time, three randomly selected seedlings per sub-plot were harvested for measurements of aboveground biomass, a total of 72 seedlings. In field, height, root collar diameter and fresh weight of the aboveground biomass of each harvested seedling were measured. Once these measurements had been acquired, a stem sample (in the form of a 10 mm thick disc) was taken from each harvested seedling along with a representative branch and a shoot from the previous year. These samples were then transported to the laboratory. In the laboratory, the fresh weight of each sample was determined and the samples were then dried to constant weight at 70°C in order to determine dry weight and moisture content of each sample. The dry weight of the aboveground biomass was determined by assuming the stem to be conical, calculating its volume on this basis, and multiplying this calculated stem volume by the measured density of the stem sample disc as follows:
![]()
where r = seedling root collar radius, h = seedling height and ρ = average disc density. The density of the sample discs was calculated by measuring their weight and volume; the mean disc density was 0.452 g cm–3 (± 0.020) No differences in disc density was found between the treatments (p = 0.525). The fresh weights of the needle and branch samples were determined in the laboratory and used in conjunction with the measured moisture contents to determine the total dry weight of the aboveground biomass (including the stem, needles and branches). A simple linear regression model was created to estimate the total aboveground biomass of all living seedlings in each plot at Hagfors, based on the dry weight of the aboveground biomass (g) and the diameter (mm) and height (mm) of the harvested seedlings. Hereafter, the model was applied on all seedlings as follows to get the seedling biomass:
![]()
where d = diameter, h = height and α = the parameter estimate derived from the model based on the sample seedlings. The R2-value of the model was 0.96 and the model was applied on all treatment combinations, i.e. pre-harvest fertilization and scarification.
At the same time as seedling sampling was performed, sampling of the ground vegetation was made at Hagfors. Ground vegetation was collected from 30 cm x 30 cm areas in the sub-plots without scarification and from the furrows, tilts, and areas between the furrows in the scarified sub-plots. Ten samples were taken from each type of soil disturbance, giving a total of 40 vegetation samples per main plot. The locations of the sampling areas within each sub-plot were selected at random. All ground vegetation samples, which included both grasses, ericaceous, herbaceous and woody species, were collected by clipping. The material was pooled into one sample for each sub-treatment per sub-plot, which gave a total of 48 samples. The dry weight of the plant material was determined after drying at 70°C to a constant weight and the samples were grinded before chemical analysis. In order to properly reflect the different micro-habitats within the scarified sub-plots, the vegetation biomass data were area–weighted. Based on the findings of Ring et al. (2013), this was done by assuming that the furrows, tilts, and areas between furrows accounted for 41%, 37%, and 21%, respectively, of the total area of the scarified sub-plots. The total amount of vegetation per fertilization regime and scarification treatment (g m–2) was calculated by summing the dry weights of the ground vegetation and the planted seedlings per m2. The weights of the seedlings were adjusted based on the observed levels of mortality in each sub-plot and the spacing of the seedlings when they were planted (i.e. 0.25 seedlings per m2).
All samples, both seedling parts and ground vegetation, were sent to the laboratory at the Department of Forest Ecology and Management, SLU, Umeå Sweden. The vegetation and needle samples were analyzed for the concentrations of Al, B, Ca, Cu, Fe, K, Mg, Mn, Na, P, S and Zn. A part of each sample was dissolved in acid under microwave heating, and the levels of the specified elements in the resulting solution were measured by ICP-AES. The elemental concentrations were determined in units of mg per g dry weight of the needle sample. Analyzes for N and C were performed for all vegetation samples and seedling parts by means of EA-IRMS, using an elemental analyzer (Flash EA 2000, Thermo Fisher Scientific) that was connected to a mass spectrometer (DeltaV, Thermo Fisher Scientific). The concentrations of N and C in the samples were specified as percentages of the dry weight of the samples. The nitrogen contents of the aboveground parts of the seedlings were calculated by multiplying the measured nitrogen concentrations of the needles, stems and branches by the dry weights of each part.
2.3 Statistical analyses
All analyses were performed using the SAS software package version 9.2 (Copyright, SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA). For each of the selected variables (seedling growth, ground vegetation and nutrient data), the analyses were based on sub-plot mean values for the fifth growing season after planting. The analyses of seedling data included data from living seedlings alone. Analyses of variance were made using the mixed model procedure, PROC MIXED, and the following model was used to analyze the data collected at Hagfors:
![]()
where µ was the general mean, φi the fixed block effect (i = 1–3), αj the fixed effect of the fertilization treatment (j = 1–4), βk the fixed effect of scarification (k = 1–2), and εijk the random error term. The interaction between block and fertilization (φα)ij was used as the error term for block and fertilization, respectively. The interaction between fertilization and scarification (αβ)jk was also included in the model and the error term for scarification and its interaction with fertilization was the mean square error. The same model was used to analyze the data from Nissafors, with the exception that only two fertilization treatments were evaluated in that case (j = 1–2). Where significant treatment differences were detected, the treatments were separated by overall pair-wise comparisons using differences of least squares means and Tukey-Kramer for the adjustment for multiple comparisons. For all tests, an α-value of 0.05 was used to indicate statistical significance.
Seedling survival and damage were analyzed using PROC GLIMMIX, assuming a binomial distribution. The model established for the growth data was used (Eq. 3), with the response variable being the number of dead or damaged seedlings divided by the total number of seedlings per block and treatment. The chi-square values of the models were 1.46 for the survival data and 1.10 for the damage data from Hagfors, and 1.02 for the Nissafors survival data.
3 Results
3.1 Seedling mortality and damage
After five growing seasons no main effects of previous fertilization treatments on seedling mortality and damage were detected, but main effects of scarification and interactions between previous fertilization and scarification were found (Table 3). Seedling mortality differed between the scarification treatments both at Hagfors and at Nissafors. At Hagfors, 96% of the planted seedlings survived in the scarified sub-plots whereas the mean survival rate for the sub-plots without scarification was 70% (Fig. 1). At Nissafors, the survival rate for seedlings planted in scarification was 90%, compared to 63% without scarification. The lower survival rate in the sub-plots without scarification at Nissafors was mainly due to higher levels of pine weevil damage, which accounted for 73% of the mortality in these plots. Scarification reduced pine weevil damage, herbivory and damage caused by unknown factors (p < 0.001 for all factors). There were significant interaction effects between scarification and previous fertilization at Hagfors, showing that fertilization increased mortality in the absence of scarification, with the highest levels of mortality occurring at the greatest dosage of N, 1800N (Table 3, Fig. 1). The higher mortality rate in the sub-plots without scarification was mainly due to damage caused by pine weevils and herbivory (Fig. 1). At Nissafors, there were no interaction effects between scarification and fertilization, but here only the lower N dosage, 450N, was included in the experiment.
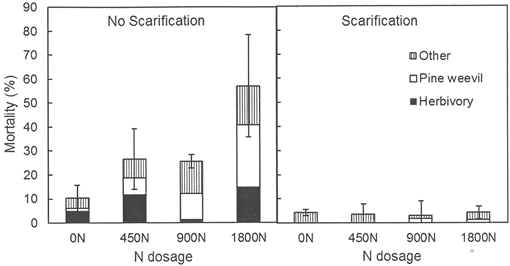
Fig. 1. Mortality (% dead seedlings) for seedlings planted in sub-plots with and without scarification at Hagfors five years after planting. Three types of damage were distinguished: damage caused by pine weevils, damage caused by herbivory, and damage caused by unknown factors. 0N, 450N, 900N and 1800N denote the applied total N dosage (in kg ha–1).
3.2 Seedling growth
No effects of previous fertilization regimes or any interactions between fertilization and scarification were found regarding seedling growth, but scarification had a significantly positive effect on seedling growth at both Hagfors and Nissafors (Table 3). The difference in growth between the scarified sub-plots and the sub-plots without scarification increased over time and five growing seasons after planting, the mean height of the seedlings planted in scarified sub-plots at Hagfors was 113 cm (Fig. 2), with a mean diameter of 4.0 cm. In sub-plots without scarification, the mean seedling height and diameter were 88 cm and 3.1 cm, respectively. The seedlings at Nissafors were slightly larger than at Hagfors. Five years after planting the seedlings planted in scarified sub-plots had a mean height of 138 cm and a mean diameter of 4.3 cm. The corresponding values for seedlings in sub-plots without scarification were 111 cm and 3.2 cm, respectively. As with height and diameter, scarification had a positive effect on aboveground seedling biomass at Hagfors (Table 3, Fig. 3). On average, the aboveground biomass for seedlings grown in scarified sub-plots was 610 g while that for seedlings in sub-plots without scarification was 324 g. When converting the values from biomass per seedling to biomass per area (m2), the effects of scarification on mortality had to be included. After this compensation, it was found that the aboveground biomass after scarification was 147 g m–2. In the absence of scarification, the aboveground biomass was 59 g m–2.
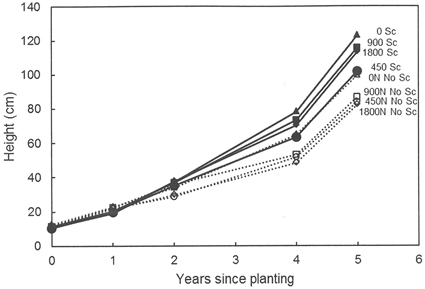
Fig. 2. Height development (mm) for seedlings grown in the different treatment combinations at Hagfors. 0N, 450N, 900N and 1800N denote the applied N dosage (in kg ha–1). No Sc = no scarification; Sc = scarification (disc trenching).
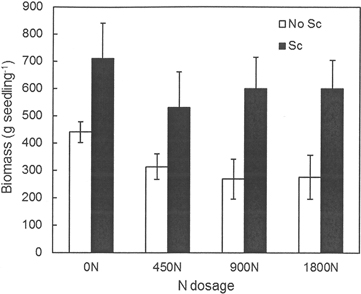
Fig. 3. Aboveground biomass (g dry weight per seedling) for seedlings grown in the different treatment combinations at Hagfors. 0N, 450N, 900N and 1800N denote the applied N dosage (in kg ha–1). No Sc = no scarification; Sc = scarification (disc trenching).
| Table 3. Results obtained in the analyses of the different response variables, shown in terms of the p-values for each treatment effect and site. | ||||||
| Site | Treatment | Variable | ||||
| Mortality | Damage | Height | Diameter | Biomass | ||
| Hagfors | Fertilization | 0.813 | 0.682 | 0.337 | 0.654 | 0.591 |
| Scarification | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Fert. * Scar. | <0.001 | <0.001 | 0.291 | 0.275 | 0.682 | |
| Nissafors | Fertilization | 0.620 | 0.577 | 0.659 | ||
| Scarification | <0.001 | 0.002 | 0.002 | |||
| Fert. * Scar. | 0.140 | 0.883 | 0.486 | |||
3.3 Seedling nutrients
Seedling nutrient data was only obtained at Hagfors. With a few exceptions, the concentrations of the different elements in the needles five years after planting did not differ significantly between previous fertilization regimes or scarification treatments (Table 4). However, the levels of aluminum (Al), boron (B) and manganese (Mn) did differ between scarification treatments with increasing needle concentration in seedlings planted after scarification. The Al concentration was 0.23 mg g–1 without scarification and 0.31 mg g–1 with scarification, and the corresponding values for B 8.54 μg g–1 and 11.8 μg g–1, and for Mn 0.37 mg g–1 and 0.47 mg g–1, respectively. For B and magnesium (Mg) there was an effect of N dosage. The B concentration increased significantly with larger previous fertilization dosages (4.33 μg g–1 at 0N, 8.50 μg g–1 at 450N, 11.0 μg g–1 at 900N and 16.8 μg g–1 at 1800N), while the effect for Mg showed a higher concentration in 900N compared to 0N. In addition, there was a significant interaction effect between fertilization and scarification for sodium (Na), with higher concentrations of Na without scarification compared to scarified sub-plots in 450N and 900N.
| Table 4. Concentrations of Al, Ca, K, Mg, Mn, P and S (mg g–1) and B, Cu, Fe, Na and Zn (μg g–1) in the needles of seedlings grown in different treatments at Hagfors and p-values for fertilization, scarification and their interaction according to Eq. 3. Needles were sampled five years after planting. No Sc = no scarification and Sc = scarification. | |||||||||||
| Element | p-values | Treatments | |||||||||
| Fert. | Scar. | Fert.*Scar. | 0N | 450N | 900N | 1800N | |||||
| No Sc | Sc | No Sc | Sc | No Sc | Sc | No Sc | Sc | ||||
| Al | 0.180 | 0.015 | 0.677 | 0.24 | 0.27 | 0.26 | 0.32 | 0.22 | 0.33 | 0.23 | 0.30 |
| B | 0.001 | 0.001 | 0.075 | 3.67 | 5.00 | 7.67 | 9.33 | 8.33 | 13.7 | 14.0 | 19.0 |
| Ca | 0.411 | 0.115 | 0.671 | 1.85 | 2.28 | 1.90 | 2.23 | 1.72 | 2.19 | 2.37 | 2.24 |
| Cu | 0.213 | 0.456 | 0.885 | 1.67 | 2.00 | 1.67 | 2.00 | 2.33 | 2.33 | 2.00 | 2.00 |
| Fe | 0.147 | 0.947 | 0.308 | 29.7 | 30.0 | 60.0 | 36.0 | 29.3 | 36.0 | 42.0 | 49.7 |
| K | 0.243 | 0.367 | 0.376 | 2.84 | 3.07 | 3.31 | 3.18 | 2.89 | 3.49 | 2.69 | 2.63 |
| Mg | 0.025 | 0.153 | 0.743 | 0.50 | 0.50 | 0.58 | 0.48 | 0.66 | 0.61 | 0.66 | 0.56 |
| Mn | 0.134 | 0.010 | 0.820 | 0.38 | 0.44 | 0.41 | 0.50 | 0.31 | 0.43 | 0.41 | 0.52 |
| Na | 0.685 | 0.237 | 0.009 | 29.3 | 24.7 | 30.3 | 24.0 | 24.3 | 33.0 | 31.5 | 29.0 |
| P | 0.758 | 0.479 | 0.148 | 0.81 | 0.83 | 0.87 | 0.83 | 0.79 | 0.94 | 0.93 | 0.87 |
| S | 0.453 | 0.800 | 0.252 | 0.54 | 0.54 | 0.61 | 0.57 | 0.53 | 0.62 | 0.68 | 0.58 |
| Zn | 0.472 | 0.306 | 0.492 | 35.0 | 33.7 | 40.7 | 34.7 | 93.3 | 34.7 | 40.5 | 38.0 |
Needle N concentrations did not differ between treatments (i.e. they were not affected by fertilization or scarification) and the overall mean N concentration was 0.80%. Similarly, no differences were found between treatments for the other seedling parts. The mean N concentration in the stem was 0.27% and that in the branches was 0.35%. The C concentration varied between 50% and 52% for all seedling parts and treatments. The total N content of the aboveground biomass of the seedlings differed between scarification treatments (p = 0.005) (Fig. 4). Seedlings planted in scarified plots had a higher N content, 2.88 g versus 1.55 g per seedling for seedlings planted without any scarification.
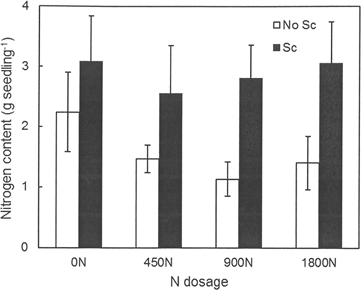
Fig. 4. Nitrogen content (g per seedling) in seedlings grown in the different treatment combinations at Hagfors. 0N, 450N, 900N and 1800N denote the applied N dosage (in kg ha–1). No Sc = no scarification; Sc = scarification (disc trenching).
Ratios between the needle concentrations (mg g–1 dry weight) of some essential elements (P, K, Ca, Mg and S) and the needle N concentrations for each treatment combination were compared (Table 5). The treatment with the greatest N dose (1800N) reduced the K:N ratio relative to that observed under the other fertilization treatments. For the Ca:N ratio, there was a significant main effect for scarification (p = 0.031), where the Ca:N ratio was higher after scarification, 0.27 compared to 0.23. The combination of previous fertilization with a dose of 900 kg N ha–1 and scarification produced somewhat higher ratios than the other investigated treatments for all of the studied elements, although these differences were not always significant (see Table 5 for details).
| Table 5. The ratios of the P, K, Ca, Mg and S concentrations to the N concentration in the needles of the seedlings grown at Hagfors in different treatments. Values followed by different letters within the same rows are significantly different. No Sc = no scarification and Sc = scarification. | ||||||||
| Ratio | Treatments | |||||||
| 0N | 450N | 900N | 1800N | |||||
| No Sc | Sc | No Sc | Sc | No Sc | Sc | No Sc | Sc | |
| P:N | 0.10a | 0.10a | 0.10a | 0.10a | 0.10a | 0.12b | 0.10a | 0.10a |
| K:N | 0.35ac | 0.38ab | 0.38ab | 0.37ac | 0.38abc | 0.44b | 0.29c | 0.31ac |
| Ca:N | 0.22 | 0.28 | 0.22 | 0.26 | 0.22 | 0.28 | 0.25 | 0.27 |
| Mg:N | 0.06ab | 0.06ab | 0.07ab | 0.06a | 0.09b | 0.08ab | 0.07ab | 0.07ab |
| S:N | 0.07a | 0.07a | 0.07a | 0.07a | 0.07a | 0.08b | 0.07ab | 0.07a |
3.4 Ground vegetation
The fertilization regime applied during the previous rotation period at Hagfors had no significant effect on the total weight of the ground vegetation and the same was true for all of its analyzed chemical constituents other than K (Table 6). The K content of the vegetation increased with the intensity of previous fertilization. Scarification significantly reduced the weight of ground vegetation per m2 (Fig. 5). In the scarified treatment (disc trenching), there was no difference in ground vegetation biomass between the furrow, the tilt or the area between furrows. No interaction between previous fertilization regime and the furrow, the tilt or the area between furrows within the scarification treatment was found. In addition, the nutrient contents of the vegetation following scarification were universally lower than those found in sub-plots without scarification. This was because of the lower total weight of the ground vegetation in scarified sub-plots. The concentrations of the measured elements did not differ between the various fertilization treatments (data not shown).
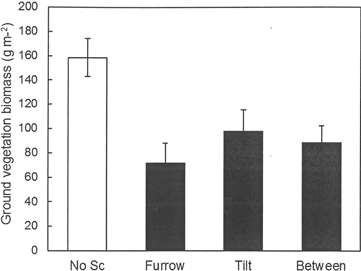
Fig. 5. Biomass of ground vegetation (g m–2) in the different scarification treatments at Hagfors. For the plots without scarification the overall mean value is shown. In the disc trenched treatment (scarification), mean values are shown for the furrow, the tilt and the area between furrows.
The total aboveground biomass, including both ground vegetation and the planted seedlings, did not differ between previous fertilization (p = 0.687) or scarification treatments (p = 0.399). Without scarification, the total aboveground biomass was 218 g m–2 whereas that in scarified sub-plots was 233 g m–2. Hence, the loss of planted seedling biomass in the sub-plots without scarification was replaced by other species. Regarding the total N-content of the aboveground biomass, it was slightly greater in sub-plots without scarification 2.21 g m–2 versus 1.72 g m–2 (p = 0.033), due to a higher N-concentration in the ground vegetation (1.20%) in comparison with the planted seedlings (0.47%). No effect of previous fertilization on total N-content was found (p = 0.191).
| Table 6. Summary of the results of the statistical analysis, shown as p-values, and the weight and chemical contents of the ground vegetation for each treatment at Hagfors (area-weighted values). The contents are given in g m–2 (for weight, C, N, Ca, K, Mg, Mn, P and S) or mg m–2 (for Al, B, Cu, Fe, Na and Zn). Values followed by different letters within the same rows are significantly different. | ||||||||||
| p-values | Treatments | |||||||||
| Fert. | Scar. | Fert.*Scar. | 0N | 450N | 900N | 1800N | No Sc | Sc | ||
| Weight | 0.425 | 0.006 | 0.901 | 112.2 | 97.6 | 132.6 | 147.8 | 158.6a | 86.49b | |
| C | 0.535 | 0.007 | 0.960 | 57.37 | 50.25 | 68.38 | 71.28 | 79.71a | 43.93b | |
| N | 0.278 | 0.003 | 0.707 | 1.227 | 1.250 | 1.589 | 1.871 | 1.943a | 1.025b | |
| Al | 0.217 | 0.058 | 0.921 | 19.47 | 19.82 | 20.82 | 11.13 | 21.38 | 14.23 | |
| B | 0.489 | 0.144 | 0.942 | 1.596 | 1.679 | 2.251 | 2.302 | 2.268 | 1.646 | |
| Ca | 0.331 | 0.006 | 0.894 | 0.800 | 0.713 | 0.938 | 0.551 | 0.989a | 0.512b | |
| Cu | 0.404 | 0.015 | 0.990 | 0.548 | 0.452 | 0.665 | 0.515 | 0.667a | 0.424b | |
| Fe | 0.962 | 0.766 | 0.698 | 8.129 | 8.895 | 7.604 | 8.037 | 8.431 | 7.902 | |
| K | 0.005 | 0.011 | 0.068 | 0.550b | 0.503b | 0.684b | 1.183a | 0.977a | 0.483b | |
| Mg | 0.356 | 0.006 | 0.985 | 0.137 | 0.125 | 0.181 | 0.175 | 0.197a | 0.113b | |
| Mn | 0.923 | 0.037 | 0.906 | 0.119 | 0.101 | 0.119 | 0.111 | 0.140a | 0.086b | |
| Na | 0.279 | 0.545 | 0.840 | 2.767 | 3.872 | 2.964 | 5.041 | 3.931 | 3.391 | |
| P | 0.266 | 0.016 | 0.648 | 0.139 | 0.133 | 0.171 | 0.205 | 0.216a | 0.108b | |
| S | 0.510 | 0.007 | 0.967 | 0.115 | 0.106 | 0.145 | 0.146 | 0.163a | 0.092b | |
| Zn | 0.705 | 0.003 | 0.937 | 3.818 | 3.239 | 4.399 | 4.018 | 5.143a | 2.596b | |
4 Discussion
Previous fertilization had no effect on seedling growth or the amount of ground vegetation. Ring et al. (2011) found that fertilization had significant effects on soil chemistry, and that concentrations and contents of N and exchangeable P and Mg increased with increasing fertilizer application rate, while the concentration and contents of K decreased. In our study, more intensive fertilization regimes caused increases in the B and Mg concentration in the needles of the planted seedlings, but did not have any significant effects on the concentration of N, P, or K (B and Mg were added with the N-fertilizer, see Table 1). On sandy soils, B deficiency can occur (Stone 1990), but no effects on seedling growth were found in this study. For all of the studied treatment combinations, the concentrations of nutrient elements (N and P in particular) in the needles were relatively low compared to those observed in previous studies (Högbom et al. 2001; Johansson et al. 2012). This could partly be explained by the fact that the needles were sampled in June, when their concentrations of carbohydrates and starch increases, and this causes a dilution effect on needle nutrient concentrations (Linder 1995). On the other hand, the ratios of macronutrient levels to N concentrations observed in this work were similar to the optimal levels reported in previous studies (Ingestad 1979; Aronsson and Elowson 1980; Linder 1995), suggesting that there were no nutrient imbalances for seedlings planted in any of the treatments. However, there was one exception to this general finding. The K:N ratio in seedlings planted in the most intense fertilization regime (1800N) was below the target value of 0.35, although it was not significantly different to that observed for the other treatments. This apparent reduction in K uptake is consistent with the findings of Ring et al. (2011), who reported that the soil K concentration and content decreased as the amount of N applied increased. The reduced abundance of K in the seedlings and soil may be partly due to increased uptake by ground vegetation, where the K content increased. Contrary to our hypothesis, there were no interactions between previous fertilization and scarification that affected the growth of seedlings or ground vegetation. But, in accordance with these results, recent results obtained by analyzing soil solution samples taken from the studied sites showed that the level of N in the soil solution was not influenced by any interactions between the applied N fertilization regime and scarification (Ring et al. 2013).
The lack of carryover effects of previous N fertilization on seedling growth and the size of the experimental plots can be discussed. Although there is a possibility of movement of nutrients between plots and that the plots were fairly small, they were distributed over a large site and the layout was in accordance with other fertilization experiments (Berch et al. 2006; Albaugh et al. 2012). Results obtained from the previous stands in the current study showing positive growth effects of the different N fertilization regimes also reduced this concern. Another aspect that could have reduced positive carryover effects of previous N fertilization in the newly established stands is the removal of logging residues on the experimental sites. In a similar study performed in the Pacific Northwest, the tops and foliage of the harvested trees were left on the sites, and positive growth effects were found on seedlings planted in previously fertilized plots (Footen et al. 2009). The retention of logging debris can increase seedling foliar nitrogen content and early growth (Harrington et al. 2013), and if the N-contents in the logging debris increased due to previous N fertilization, there might have been a positive growth effect if they were left on the experimental sites.
As expected, scarification significantly increased the survival and growth of the planted seedlings five years after clear-felling at both Hagfors and Nissafors. With scarification, in this case disc trenching, the microsite was improved and the amount of competing ground vegetation reduced. By increasing nutrient and water availability, by reducing competition from vegetation during the period immediately following planting and also by reducing various types of damage, scarification has been shown to improve the microsite, and thus seedling growth many times before (Örlander et al. 1990; Prevost 1992; Nilsson and Örlander 1999; Löf et al. 2012; Johansson et al. 2013). Not surprisingly, most of the damage and mortality that occurred in the treatment without scarification was caused by pine weevil. Even though the seedlings were treated with insecticides prior to planting and during the following season, damage can occur if seedlings are not planted in pure mineral soil (Petersson et al. 2005; Luoranen and Viiri 2012). The observation of interactions between scarification and the fertilization regime applied during the preceding rotation period demonstrated that higher intensities of fertilization were associated with increased damage (caused by pine weevils, large herbivores, and other factors) and mortality in sub-plots without scarification at Hagfors. Fertilization at the time of planting has been shown to increase insect damage and in a study performed by Zas et al. (2006) it was found that fertilization increased the severity of pine weevil attacks to the point that the growth gains due to the fertilizer treatment was outweighed by the increased damage. The increase in the amount of ground vegetation in sub-plots without scarification and a weak tendency towards even more vegetation in fertilized plots (900N and 1800N) could be another explanation to the higher level of damage in these treatments. The presence of larger quantities of vegetation increases the likelihood of seedling damage, probably because the vegetation provides shelter for pine weevils (Petersson et al. 2006). Also, the composition of the ground vegetation seemed to be different in these treatments with more grass on fertilized plots; however this was only visually observed and not measured in the study. This difference in vegetation composition might have changed the competition dynamics, leading to reduced seedling vitality and a higher level of mortality caused by unknown factors. Another important cause of damage at Hagfors was herbivory by large animals. These herbivores may have been attracted by an additional food source in the fertilized plots without scarification. It has been shown that there is a significant positive correlation between browsed biomass and the total biomass available for browsing (Bergqvist et al. 2012) and that fertilization increases browsing (Månsson et al. 2009). Also, the total biomass N content was also higher in sub-plots without scarification. It has been shown that the level of browsing increases with needle N concentration (Bergquist and Örlander 1998), and this may hold true for other biomass as well. Although there were no significant differences in the needle N concentrations or the nutrient composition of the seedlings examined in this study, fertilization could conceivably have changed the nutritional quality or the secondary metabolite concentrations of the seedlings in a way that we did not measure, but which could have attracted both insects and large herbivores (Kytö et al. 1996; Stolter et al. 2009).
Scarification increased the growth of the planted seedlings. At both Hagfors and Nissafors, scarification significantly increased the concentration of nitrate NO3- - N below the tilts created by disc trenching (Ring et al. 2013). Other studies have also shown that disc trenching increases mineralization and the availability of soil nutrients (Lundmark-Thelin and Johansson 1997). Although the seedlings were planted in the furrow right next to the tilt, the seedlings’ root systems probably reached the pool of N in the tilt shortly after they were planted. As suggested by Tanskanen and Ilvesniemi (2007), the tilt is probably the part from where most of the uptake of nutrient and water is taking place. In the treatment with scarification, there was no significant difference between the ground vegetation biomass in the furrow, tilt or in the area between the furrows, thus indicating that soil scarification only reduced competing vegetation in the planting spots during the first few years after implementation. However, at that time the pine seedlings were large enough to suppress the competing vegetation in the scarified plots. Interestingly, the total quantity of above ground biomass, including both planted seedling and ground vegetation, was similar in both sub-plots with and without scarification. This shows that scarification caused a shift in the vegetation dynamics, and that reductions in the biomass of the planted seedlings were replaced by other species when scarification was not performed. This study only investigated the short-term effects of scarification; it is possible that its positive effects on growth and survival may be even more pronounced in the long term. After accounting for differences in mortality rates, scarification increased the aboveground seedling biomass by a factor of 2.5 relative to that achieved in sub-plots without scarification five years after planting. A difference of this magnitude would be expected to affect the future development of the stand. Other studies have shown that scarification increases volume production over longer periods of time (Mattsson and Bergsten 2003) and that it reduces the variation in the heights of the planted trees, creating a more homogeneous stand (Johansson et al. 2013). On the other hand, scarification might increase competition from other, naturally regenerated tree species in the long run because it creates an environment that is favorable for establishment in general rather than one that is selective for the desired species (Lehtosalo et al. 2010; Johansson et al. 2013).
In conclusion, pre-harvest fertilization had few effects on the planted seedlings and the ground vegetation. Scarification had a significant and positive effect on seedling survival and growth. It reduced the abundance of ground vegetation, thus increasing the availability of site resources for the seedlings. Seedlings planted in sub-plots without scarification and with larger pre-harvest fertilization doses exhibited lower survival rates than those subjected to other treatments. This may have been because undisturbed soil and heavy fertilization created an environment that was more favorable for large herbivores and pine weevils, or caused changes in seedling chemistry that was not detected in this study. Also, changes in competition dynamics between ground vegetation and planted seedlings could be another explanation. More data would be required to reliably determine the cause of these effects. The two experimental sites were located on sandy soils with relatively low productivity and it is possible that different results would have been obtained on other soil types.
Acknowledgements
The establishment of the study, the measurement and the analyses was financed by Formas and Stiftelsen Svensk Växtnäringsforskning. K. Johansson was partially supported by a grant from Vinnova (TC4F – Trees and Crops for the Future) and Skogforsk. The contribution by E. Ring was financed by Future Forests, a multi-disciplinary research program supported by the Foundation for Strategic Environmental Research, Swedish Forestry, SLU, Umeå University and Skogforsk. The authors are thankful to the owners of the forest sites examined in this work – StoraEnso and Bergvik (Hagfors) and Sveaskog (Nissafors). We would also like to thank all of the staff at both Skogforsk and SLU who conducted the field work discussed herein: Lars-Åke Dahl, Emma Dahl, Karolina Dahl, Thomas Hjerpe, Helena Lundhammar, Sten Nordlund, Martin Rappe-George, Kjell Rosén, and Rolf Övergaard.
References
Albaugh T.J., Stape J.L., Fox T.R., Rubilar R.A., Allen H.L. (2012). Midrotation vegetation control and fertilization response in Pinus taeda and Pinus elliottii across the Southeastern United States. Southern Journal of Applied Forestry 36: 44–53. http://dx.doi.org/http://dx.doi.org/10.5849/sjaf.10-042.
Aronsson A., Elowson S. (1980). Effects of irrigation and fertilization on mineral nutrient in scots pine seedlings. Ecological Bulletins 32: 219–228.
Berch S.M., Brockley R.P., Battigelli J.P., Hagerman S., HollBerch B. (2006). Impacts of repeated fertilization on components of the soil biota under a young lodgepole pine stand in the interior of British Columbia. Canadian Journal of Forest Research 36: 1415–1426. http://dx.doi.org/10.1139/X06-037.
Bergh J., Linder S., Lundmark T., Elfving B. (1999). The effect of water and nutrient availability on the productivity of Norway spruce in northern and southern Sweden. Forest Ecology and Management 119: 51–62. http://dx.doi.org/10.1016/S0378-1127.
Bergquist J., Örlander G. (1998). Browsing damage by roe deer on Norway spruce seedlings planted on clearcuts of different ages: 2. Effect of seedling vigour. Forest Ecology and Management 105: 295–302. http://dx.doi.org/10.1016/S0378-1127.
Bergqvist G., Bergström R., Wallgren M. (2012). Browsing by large herbivores on Scots pine (Pinus sylvestris) seedlings in mixture with ash (Fraxinus excelsior) or silver birch (Betula pendula). Scandinavian Journal of Forest Research 27: 372–378. http://dx.doi.org/10.1080/02827581.2011.635155.
Brand D.G. (1991). The establishment of boreal and sub-boreal conifer plantations: an integrated analysis of environmental conditions and seedling growth. Forest Science 37: 68–100.
Day K.R., Leather S. (1997). Threats to forestry by insect pests in Europe. In: Watt A.D., Stork N.E., Hunter M.D. (eds.). Forests and insects. Chapman and Hall, UK. p. 177–205.
Footen P.W., Harrison R.B., Strahm B.D. (2009). Long-term effects of nitrogen fertilization on the productivity of subsequent stands of Douglas-fir in the Pacific Northwest. Forest Ecology and Management 258: 2194–2198. http://dx.doi.org/10.1016/j.foreco.2009.02.033.
Grossnickle S.C. (2000). Ecophysiology of northern spruce species: the performance of planted seedlings. NRC Research Press, Ottawa, Ontario, Canada. 409 p.
Harrington T.B., Slesak R.A., Schoenholtz S.H. (2013). Variation in logging debris cover influences competitor abundance, resource availability, and early growth of planted Douglas-fir. Forest Ecology and Management 296: 41–52. http://dx.doi.org/10.1016/j.foreco.2013.01.033.
Hedwall, P-O., Nordin A., Brunet J., Bergh J. (2010). Compositional changes of forest-floor vegetation in young stands of Norway spruce as an effect of repeated fertilisation. Forest Ecology and Management 259: 2418–2425. http://dx.doi.org/10.1016/j.foreco.2010.03.018.
Högbom L., Nohrstedt, H-Ö, Lundström H., Nordlund S. (2001). Soil conditions and regeneration after clear-felling of a Pinus sylvestris L. stand in a nitrogen experiment, Central Sweden. Plant and Soil 233: 241–250. http://dx.doi.org/10.1023/A:1010556825915.
Ingestad T. (1979). Mineral nutrient requirements of Pinus sylvestris and Picea abies seedlings. Physiologia Plantarum 45: 373–380. http://dx.doi.org/10.1111/j.1399-3054.1979.tb02599.x.
Jacobson S., Pettersson F. (2010). An assessment of different fertilization regimes in three boreal coniferous stands. Silva Fennica 44: 815–827.
Johansson K., Nilsson U., Allen H.L. (2007). Interactions between soil scarification and Norway spruce seedling types. New Forests 33: 13–27. http://dx.doi.org/10.1007/s11056-006-9010-y.
Johansson K., Langvall O., Bergh J. (2012). Optimization of environmental factors affecting initial growth of Norway spruce seedlings. Silva Fennica 46: 27–38.
Johansson K., Nilsson U., Örlander G. (2013). A comparison of long-term effects of scarification methods on the establishment of Norway spruce. Forestry 86: 91–98. http://dx.doi.org/10.1093/forestry/cps062.
Lehtosalo M., Mäkelä A., Valkonen S. (2010) Regeneration and tree growth dynamics of Picea abies, Betula pendula and Betula pubescens in regeneration areas treated with spot mounding in southern Finland. Scandinavian Journal of Forest Research 25: 213–223. http://dx.doi.org/10.1080/02827581.2010.489514.
Linder S. (1995). Foliar analyses for detecting and correcting nutrient imbalances in Norway spruce. Ecological Bulletins 44: 178–190.
Löf M., Dey D.C., Navarro R.M., Jacobs D.F. (2012). Mechanical site preparation for forest restoration. New Forests 43: 825–848. http://dx.doi.org/10.1007/s11056-012-9332-x.
Lundmark-Thelin A., Johansson, M-B. (1997). Influence of mechanical site preparation on decomposition and nutrient dynamics of Norway spruce (Picea abies (L.) Karst.) needle litter and slash needles. Forest Ecology and Management 96: 101–110. http://dx.doi.org/10.1016/S0378-1127(97)00040-6.
Luoranen J., Viiri H. (2012). Soil preparation reduces pine weevil (Hylobius abietis (L.)) damage on both peatland and mineral soil sites one year after planting. Silva Fennica 46: 151–161.
Månsson J., Bergström R., Danell K. (2009). Fertilization – effects on deciduous tree growth and browsing by moose. Forest Ecology and Management 258: 2450–2455. http://dx.doi.org/10.1016/j.foreco.2009.08.025.
Mattsson S., Bergsten U. (2003) Pinus contorta growth in northern Sweden as affected by soil scarification. New Forests 26: 217–231. http://dx.doi.org/10.1023/A:1024425205712.
Melin J., Nômmik H. (1988). Fertilizer nitrogen distribution in a Pinus sylvestris/Picea abies ecosystem, Central Sweden. Scandinavian Journal of Forest Research 3: 3–15. http://dx.doi.org/10.1080/02827588809382490.
Munson A.D., Margolis H.A., Brand D.G. (1993). Intensive silvicultural treatment – impacts on soil fertility and planted conifer response. Soil Science Society of America Journal 57: 246–255.
Nilsson U., Allen H.L. (2003). Short- and long-term effects of site preparation, fertilization and vegetation control on growth and stand development of planted loblolly pine. Forest Ecology and Management 175: 367–377. http://dx.doi.org/10.1016/S0378-1127(02)00140-8.
Nilsson U., Örlander G. (1999). Water uptake by planted Picea abies in relation to competing field vegetation and seedling rooting depth on two grass-dominated sites in southern Sweden. Scandinavian Journal of Forest Research 14: 312–319. http://dx.doi.org/10.1080/02827589950152629.
Nohrstedt H.Ö. (1990). Effects of repeated nitrogen fertilization with different doses on soil properties in a Pinus sylvestris stand. Scandinavian Journal of Forest Research 5: 3–15. http://dx.doi.org/10.1080/02827589009382588.
Nohrstedt H.Ö. (2001). Response of coniferous forest ecosystems on mineral soils to nutrient additions: a review of Swedish experiences. Scandinavian Journal of Forest Research 16: 555–573. http://dx.doi.org/10.1080/02827580152699385.
Nordlander G., Hellqvist C., Johansson K., Nordenhem H. (2011). Regeneration of European boreal forests: effectiveness of measures against seedling mortality caused by the pine weevil Hylobius abietis. Forest Ecology and Management 262: 2354–2363. http://dx.doi.org/10.1016/j.foreco.2011.08.033.
Olsson B.A., Kellner O. (2006). Long-term effects of nitrogen fertilization on ground vegetation in coniferous forests. Forest Ecology and Management 237: 458–470. http://dx.doi.org/10.1016/j.foreco.2006.09.068.
Örlander G., Gemmel P., Hunt J. (1990). Site preparation: a Swedish overview. FRDA Report 105. Canada-BC Forest Resource Development Agreement. 62 p.
Petersson M., Örlander G., Nordlander G. (2005). Soil features affecting damage to conifer seedlings by the pine weevil Hylobius abietis. Forestry 78: 83–92. http://dx.doi.org/10.1093/forestry/cpi008.
Petersson M., Nordlander G., Örlander G. (2006). Why vegetation increases pine weevil damage: bridge or shelter? Forest Ecology and Management 225: 368–377. http://dx.doi.org/10.1016/j.foreco.2006.01.012.
Pettersson F. (1994). Predictive functions for calculating the total response in growth to nitrogen fertilization, duration and distribution over time. Skogforsk Report 4. The Forestry Research Institute of Sweden, Uppsala, Sweden. ISSN 1103-6648.
Prevost M. (1992). Effects of scarification on soil properties, seedling growth and competition – review of current knowledge and research perspectives in Quebec. Annales des Sciences Forestières 49: 277–296. http://dx.doi.org/10.1051/forest:19920306.
Regeringens proposition. (2008). En skogspolitik i takt med tiden. Regeringens proposition 2007/08:108.
Ring E. (2004). Experimental N fertilization of Scots pine: effects on soil-solution chemistry 8 years after final felling. Forest Ecology and Management 188: 91–99. http://dx.doi.org/10.1016/j.foreco.2003.07.020.
Ring E., Jacobson S., Högbom L. (2011). Long-term effects of nitrogen fertilization on soil chemistry in three Scots pine stands in Sweden. Canadian Journal of Forest Research 41: 279–288. http://dx.doi.org/10.1139/X10-208.
Ring E., Högbom L., Jansson G. (2013). Effects of previous nitrogen fertilization on soil-solution chemistry after final felling and soil scarification at two nitrogen-limited forest sites. Canadian Journal of Forest Research 43: 396–404. http://dx.doi.org/10.1139/cjfr-2012-0380.
Sikström U. (2005). Pre-harvest soil acidification, liming or N fertilization did not significantly affect the survival and growth of young Norway spruce. Silva Fennica 39: 341–349.
Stolter C., Niemelä P., Ball J.P., Julkunen-Tiitto R., Vanhatalo A., Danell K. Varvikko T., Ganzhorn J.U. (2009). Comparison of plant secondary metabolites and digestibility of three different boreal coniferous trees. Basic and Applied Ecology 10: 19–26. http://dx.doi.org/10.1016/j.baae.2007.12.001.
Stone E.L. (1990). Boron deficiency and excess in forest trees: a review. Forest Ecology and Management 37: 49–75. http://dx.doi.org/org/10.1016/0378-1127(90)90046-E.
Strengbom J., Nordin A. (2008). Commercial forest fertilization causes long-term residual effects in ground vegetation of boreal forests. Forest Ecology and Management 256: 2175–2181. http://dx.doi.org/10.1016/j.foreco.2008.08.009.
Tamm C.O. (1991). Nitrogen in terrestrial ecosystmes. Questions of productivity, vegetational changes, and ecosystem stability. Ecological Studies Volume 81. Springer. 116 p. ISBN 978-3-642-75170-7.
Tanskanen N., Ilvesniemi H. (2007). Spatial distribution of fine roots at ploughed Norway spruce forest sites. Silva Fennica 41: 45–54.
Thiffault N., Titus B.D., Munson A.D. (2005). Silvicultural options to promote seedling establishment on Kalmia-Vaccinium-dominated sites. Scandinavian Journal of Forest Research 20: 110–121. http://dx.doi.org/10.1080/02827580510008356.
Zas R., Sampedro L., Prada E., Lombardero M.J., Fernández-López J. (2006). Fertilization increases Hylobius abietis L. damage in Pinus pinaster Ait. seedlings. Forest Ecology and Management 222: 137–144. http://dx.doi.org/10.1016/j.foreco.2005.10.008.
Total of 50 references

