Diversifying clearcuts with green-tree retention and woody debris structures: conservation of mammals across forest ecological zones
Sullivan T. P., Sullivan D. S. (2014). Diversifying clearcuts with green-tree retention and woody debris structures: conservation of mammals across forest ecological zones. Silva Fennica vol. 48 no. 5 article id 1219. https://doi.org/10.14214/sf.1219
Highlights
- Species diversity of small mammals increased with structural complexity left on clearcut sites
- Productivity of red-backed vole populations was higher in sites with green-tree retention (GTR) and windrows of woody debris
- GTR and windrows may provide additive effect for providing habitat to conserve mammals on clearcuts.
Abstract
We tested the hypotheses (H) that on newly clearcut-harvested sites, (H1) abundance and species diversity of the forest-floor small mammal community, and (H2) abundance, reproduction, and recruitment of red-backed voles (Myodes gapperi Vigors), would increase with higher levels of structural retention via green-tree retention (GTR) and woody debris (dispersed and constructed into windrows). Study areas were located in three forest ecological zones in southern British Columbia, Canada. For H1, mean total abundance did generally increase with the gradient of retained habitat structure. Mean species richness and diversity were similar among treatment sites but did show an increasing gradient with structural compexity. For H2, mean abundance, reproduction, and recruitment of M. gapperi were higher in GTR and windrow sites than those without retained structures. There was a positive relationship between mean abundance of M. gapperi and total volume of woody debris across treatments. This study is the first investigation of the responses of forest-floor small mammals to an increasing gradient of retained habitat structure via GTR and woody debris on clearcuts. Our assessment of a combination of these two interventions suggested a potentially strong additive effect that could be cautiously extrapolated across three forest ecological zones. With the advent of low levels of GTR on clearcuts, woody debris structures should help provide some habitat to conserve forest mammals on harvest openings.
Keywords
clearcutting;
green-tree retention;
small mammals;
coniferous forests;
ecological zones;
Myodes gapperi;
population dynamics;
red-backed voles;
woody debris structures
-
Sullivan,
Department of Forest and Conservation Sciences, Faculty of Forestry, University of BC, 2424 Main Mall, Vancouver, BC, Canada V6T 1Z4
E-mail
tom.sullivan@ubc.ca

- Sullivan, Applied Mammal Research Institute, 11010 Mitchell Avenue, Summerland, BC, Canada V0H 1Z8 E-mail dru.sullivan@appliedmammal.com
Received 3 July 2014 Accepted 23 October 2014 Published 31 December 2014
Views 204569
Available at https://doi.org/10.14214/sf.1219 | Download PDF

1 Introduction
Clearcutting of temperate zone forests is the dominant harvesting system in much of North America and northern Europe. However, maintaining mammal diversity, as a component of biodiversity is a major conservation goal in commercial forest landscapes. An array of harvesting systems that leaves residual live trees after harvest (green-tree retention (GTR)) has evolved to help achieve that goal (Franklin et al. 1997; Rosenvald and Lohmus 2008). Retention patterns may be dispersed or aggregated across harvest units, often including seed-tree, shelterwood, patch, and selection cutting systems and their variants (Smith 1986; Larsen 1995; Franklin et al. 1997). These alternative harvesting systems are more aligned with natural processes by retaining large live trees, snags, and downed logs after harvest (Franklin et al. 1997, 2002; Bunnell and Dunsworth 2009). Conservation of residual live trees increases structural diversity and provides attributes of mature forest habitat that develop sooner than in sites managed by clearcutting (McComb et al. 1993; Franklin et al. 2000).
Woody debris on the forest floor is another potential source of habitat creation on new clearcuts. It accumulates as a result of stand development and by natural (wildfire, insect outbreaks, wind events) and human-caused disturbances such as harvesting (Spies et al. 1988). Woody debris provides many important functions that are essential to maintaining biodiversity and long-term ecosystem productivity such as the slow release of nutrients “tied up” in the fines of post-harvest slash and the contribution of organic matter to soil structure and modification of near-ground microclimate (Harmon et al. 1986; McComb and Lindenmayer 1999; Bunnell and Houde 2010). Excess woody debris from felling operations is typically burned to reduce a perceived fire hazard. However, woody debris contributes to habitat quality for a wide range of mammal species (McComb 2003).
Ecological indicators of significant change in forest structure and function include terrestrial small mammals which are widespread across temperate and boreal forest ecosystems (Carey and Harrington 2001; Ecke et al. 2001, 2002; Pearce and Venier 2005). These functions include prey for many predators (Martin 1994), consumers of plants, plant products (Carey et al. 1999), and invertebrates (Gunther et al. 1983), and dispersal of fungal spores (Maser et al. 1978). Responses to clearcutting in North America are species-specific with generalists that occupy a variety of habitats such as the deer mouse (Peromyscus maniculatus Wagner), northwestern chipmunk (Neotamias amoenus Allen), and Microtus voles persisting on clearcuts, although some for variable periods. Specialists such as the southern red-backed vole (Myodes gapperi Vigors) require closed-canopy forest and disappear on clearcuts, often within a year after harvest, at least in western North America (Fisher and Wilkinson 2005; Zwolak 2009). Thus, M. gapperi is an important indicator species of closed-canopy forest conditions in managed landscapes. This microtine commonly inhabits late successional coniferous and deciduous forests across temperate and boreal North America (Merritt 1981), and hence is a good candidate species for evaluation of the development of “old forest” structural attributes in young stands. The presence of red-backed vole populations at mature or old-growth “forest” levels of abundance suggests that networks of food sources and predators (e.g., short-tailed weasel (Mustela erminea L.) and American marten (Martes americana Turton) may also be present as components of biodiversity.
Suitable habitat conditions for red-backed voles have been maintained in partially cut forests with GTR levels of > 15 m2/ha BA or 30% uncut forest across a variety of forest ecosystems as reviewed by Sullivan et al. (2008). However, responses of M. gapperi to GTR levels to these and lower thresholds have been variable and short-term (≤ 3 years post-harvest) (Sullivan et al. 2008). Gitzen et al. (2007) also noted that some of this variability may be related to flexibility in habitat occupancy and changes in forest-floor conditions. Although these partial cutting results are encouraging for maintenance of M. gapperi, clearcutting still dominates as a harvesting system, even with some degree of GTR. Thus, we ask: Is there a habitat creation approach that could be combined with relatively modest levels of GTR (i.e., < 5 m2/ha BA or 5% uncut forest) to diversify clearcuts? Some studies indicate that M. gapperi seem to select forest sites with large amounts of woody debris that moderate moisture, temperature, and cover for foraging (Tallmon and Mills 1994; Vanderwel et al. 2010; Fauteux et al. 2012). To this end, construction of piles and windrows of woody debris on new clearcuts has significantly enhanced populations of forest-floor small mammals, including M. gapperi, and some of their predators (Sullivan et al. 2012). Thus, could a combination of low, but common, levels of GTR and woody debris structures enhance habitat conditions for M. gapperi and the overall forest-floor small mammal community in newly clearcut sites?
We tested the hypotheses (H) that on newly clearcut sites, (H1) abundance and species diversity of the forest-floor small mammal community, and (H2) abundance, reproduction, and recruitment of M. gapperi, will increase with higher levels of structural retention via GTR and woody debris. A third hypothesis (H3) predicted that the above response variables on sites with GTR and windrows of debris would be comparable to or higher than those in uncut mature/old growth forest.
2 Methods
2.1 Study areas
Three replicate study areas (blocks) were located in the southern interior of British Columbia (BC), Canada: 1) East Munro (49°40´N, 119°51´W) and 2) Kathleen Lake (49°44´N, 120°06´W) on the Okanagan Plateau 25 and 47 km, respectively, west of Summerland, BC, and 3) Blaeberry River (51°28´N, 116°58´W) in the Rocky Mountains 20 km northeast of Golden, BC. Biogeoclimatic ecological zones were 1) Interior Douglas-fir (IDFdk), 2) Montane spruce (MSdm), and 3) Interior Cedar-Hemlock (ICHmk) (Meidinger and Pojar 1991).General topography at 1) and 2) is rolling hills at 1300–1520 m elevation, and at 3) ranges from hilly to steep terrain at 870–890 m elevation.
The upper IDF and MS have a cool, continental climate with cold winters and moderately short, warm summers. The average temperature is below 0°C for 2–5 months, and above 10 °C for 2–5 months, with mean annual precipitation ranging from 300 to 900 mm. Open to closed mature forests of Douglas-fir (Pseudotsuga menziesii (Mirbel) Franco. var glauca (Beissn.) Franco) cover much of the IDF zone, with even-aged post-fire lodgepole pine (Pinus contorta Dougl. ex Loud. var. latifolia Engelm.) stands at higher elevations. The MS landscape has extensive young and maturing seral stages of Pinus contorta, which have regenerated after wildfire. Hybrid interior spruce (Picea glauca × P. engelmannii (Moench) Voss) and subalpine fir (Abies lasiocarpa (Hook.) Nutt.) are the dominant shade-tolerant climax trees. Pseudotsuga menziesii is an important seral species in zonal ecosystems and is a climax species on warm south-facing slopes in the driest ecosystems. Trembling aspen (Populus tremuloides Michx.) is a common seral species and black cottonwood (Populus trichocarpa T. & G.) occurs on some moist sites (Meidinger and Pojar 1991). The ICH has an interior, continental climate with cool wet winters and warm dry summers. Mean annual temperature ranges from 2 to 8.7 °C. The temperature averages below 0 °C for 2–5 months and above 10 °C for 3–5 months of the year. Mean annual precipitation is 500–1200 mm, 25–50% of which falls as snow. Upland coniferous forests dominate the ICH landscape and comprise the highest diversity of tree species of any zone in BC. Western red cedar (Thuja plicata Donn ex D. Don) and western hemlock (Tsuga heterophylla (Raf.) Sarg.) dominate mature climax forests with Pseudotsuga menziesii, Pinus contorta, Picea glauca, Picea engelmannii, their hybrids, and A. lasiocarpa common in these stands (Meidinger and Pojar 1991).
Prior to harvesting, stands at study areas 1) and 2) were composed of a mixture of Pinus contorta with variable amounts of Pseudotsuga menziesii, Picea, and some A. lasiocarpa, and at area 3) primarily Pseudotsuga menziesii with some of the other coniferous species. Mean ages of Pinus contorta ranged from 80 to 120 years and for Pseudotsuga menziesii ranged from 120 to 220 years. Mean tree heights ranged from 10.5 to 19.5 m for Pinus contorta and from 16.7 to 27.5 m for Pseudotsuga menziesii. There were no site preparation treatments on any of these harvested sites, prior to planting. Mean area of sites ranged from 4.5 to 5.8 ha.
2.2 Experimental design
Initially, each of the three study areas was considered a regional replicate (block) with a randomized complete block design of the following five treatments (all harvested sites clearcut): (a) no GTR or windrows of woody debris (dispersed debris only); (b) GTR (5–15 trees/ha) without windrows of woody debris (c) no GTR but with woody debris distributed into windrows, (d) GTR (5–15 trees/ha) and woody debris distributed into windrows, and (e) uncut mature/old-growth forest (Fig. 1). The 15 sites (5 treatments × 3 replicates) were selected on the basis of operational scale, harvest sites that were the size of typical forestry operations, and reasonable proximity of sites to one another within a study area. However, there was a significant treatment by block interaction indicating that each block (study area) and its constituent treatments should be analyzed separately as outlined in the statistical analysis. All treatments within a study area were reasonably separated to enhance statistical independence: East Munro a mean (±SE) of 0.31 ± 0.07 km; Kathleen a mean (±SE) of 0.40 ± 0.09 km; and Blaeberry a mean (±SE) of 0.23 ± 0.03 km. A measure of this independence was that no M. gapperi were captured on more than one trapping line.

Fig. 1. Photographs (fall 2009) of treatment sites (a) dispersed woody debris, (b) dispersed woody debris with green-tree retention, (c) windrows of woody debris, (d) windrows of woody debris with green-tree retention, and (e) uncut old forest.
2.3 GTR and woody debris treatments
Timber harvesting at East Munro and Kathleen was targeted at Pinus contorta salvage after, or impending, mountain pine beetle (Dendroctonus ponderosae Hopk.) attack; at Blaeberry the target was primarily Pseudotsuga menziesii. Harvesting system was clearcut with no residual timber, and clearcut with reserves of Pseudotsuga menziesii trees as GTR units. Harvesting and subsequent woody debris treatments were installed in autumn 2009 at all study areas. Windrows were created during processing of cut timber, or by site preparation work with an excavator. At East Munro and Kathleen, windrows averaged 2–3 m in height and 7–9 m in diameter or width. At Blaeberry, windrows were smaller than the other areas, averaging 1–2 m in height and 6–7 m in width. Volumes of downed wood (≥ 1 cm in diameter) in the dispersed treatments were measured using the line-intersect method of Van Wagner (1968) in three plots, each of which was an equilateral triangle with 20 m sides. Location of plots was randomly chosen along the small mammal sampling line on each site. Volume of woody debris in a windrow was measured by the method of Hardy (1996), first estimating the volume of a window and then using the method discussed in Sullivan et al. (2011).
2.4 Small mammals
Forest-floor small mammals were sampled at 4-week intervals from May to October 2010, 2011, and 2012 at East Munro and Kathleen, and at 4- to 8-week (2010–2012) intervals at Blaeberry. Each of the 15 sites had a 143-m transect for efficient sampling of community composition of small mammals (Pearson and Ruggiero 2003). Each transect had 10 trap stations at 14.3-m intervals with four Longworth live-traps at each station. Traps were supplied with whole oats, a slice of carrot, and cotton as bedding. Each trap had a 30-cm × 30-cm plywood cover for protection from sunlight (heat) and precipitation. Traps were set on the afternoon of day 1, checked on the morning and afternoon of day 2 and morning of day 3, and then locked open between trapping periods. Small mammal species sampled by this procedure included the southern red-backed vole, long-tailed vole (Microtus longicaudus Merriam), meadow vole (M. pennsylvanicus Ord), heather vole (Phenacomys intermedius Merriam), deer mouse, northwestern chipmunk, montane shrew (Sorex monticolus Merriam), masked shrew (S. cinereus Kerr), and short-tailed weasel. All animals captured (except shrews and weasels) were ear-tagged with serially numbered tags, breeding condition noted, weighed on Pesola spring balances, and point of capture recorded (Krebs et al. 1969). The duration of the breeding season was noted by palpation of male testes and the condition of mammaries of the females (Krebs et al. 1969). Animals were released on the lines immediately after processing. The overnight trapping technique resulted in a high mortality rate for shrews. Therefore, shrews were collected, frozen, and later identified according to tooth patterns (Nagorsen 1996). All handling of animals was in accordance with the Animal Care Committee, University of British Columbia.
2.5 Demographic and diversity parameters
Abundance estimates of animals were derived from the Jolly-Seber (J-S) stochastic model for open populations with small sample size corrections (Seber 1982; Krebs 1999). The J-S model assumes marked and unmarked animals have the same capture probability in each sampling session (Krebs 1999). Because our traps were locked open between trap sessions, we expected that trap responses of animals dissipated between our monthly sampling sessions. The reliability of the Jolly-Seber model declines when population sizes are very low and no marked animals are captured (Seber 1982). Number of individual animals captured was used as the population estimate for the first and last sampling weeks, and for the enumeration of the relatively less common meadow vole, heather vole, the two shrew species, and the short-tailed weasel. Jolly trappability of M. gapperi was calculated according to the estimate discussed by Krebs and Boonstra (1984). There were three summer (May-September) and two winter (October-April) periods.
Red-backed voles were classified by age when the body mass of obvious juveniles when first captured was compared with the mass of these animals as they recruited into the population and first showed signs of sexual maturity: juvenile = 1–18 g; adult ≥ 19 g. Juveniles were considered to be young animals recruited during the study. Measurements of recruitment (new animals that entered the population through reproduction and immigration), number of successful pregnancies, and early juvenile survival were derived from the sample of animals captured in each trapping session and then summed for each summer period. A pregnancy was considered successful if a female was lactating during the period following the estimated time of birth of a litter. Early juvenile survival is an index relating recruitment of young into the trappable population to the number of lactating females (Krebs et al. 1969). A modified version of this index is number of juvenile animals at week t divided by the number of lactating females caught in week t.
Species richness was the total number of species sampled for the small-mammal community in each site (Krebs 1999). Species diversity was calculated using the estimated abundance of each species for a given sampling session and then averaged over the total sampling sessions for each year. This measure of diversity was based on the Shannon-Wiener index which is well represented in the ecological literature (Magurran 2004).
2.6 Statistical analysis
A repeated-measures analysis of variance (RM-ANOVA) (SPSS 21.0 SPSS Institute Inc., 2013) was used to determine the effect of GTR and woody debris treatments on mean values of total abundance, species richness, and species diversity of the small mammal communities among the five treatments at each of the three study areas. There were five temporal samples (May to September) for each of the three years to compare treatments at each of the Munro and Kathleen study areas. There were three temporal samples (May, July, and September) for each of the three years to compare treatments at the Blaeberry study area. This same analysis tested for differences in mean values of abundance, number of recruits, number of successful pregnancies, and index of juvenile survival for the red-backed vole. The latter two attributes were compared among treatments at Munro and Kathleen areas only, as there were insufficient data at Blaeberry. A randomized block two-way ANOVA-Model III (Zar 1999) with factor treatment as a fixed effect and factor block as a random effect was used to detect differences in dimensions of windrows among treatments. Where necessary, data were transformed (log10) to better approximate normal distribution and homogeneity of variance as measured by the Levene statistic (Fowler et al. 1998). Transformed datasets included abundance, recruits, and pregnancies for red-backed voles. Mauchly’s W-test statistic was used to test for sphericity (independence of data among repeated measures) (Littel 1989; Kuehl 1994). For data found to be correlated among years, the Huynh-Feldt (H-F) correction was used to adjust the degrees of freedom of the within-subjects F-ratio (Huynh and Feldt 1976). Logarithmic (ln based) regression analyses were conducted to determine the relationship and upper limits between mean total abundance, species richness, and species diversity of small mammals, as well as mean abundance of M. gapperi, with total volume of woody debris (dispersed and windrow treatments with and without GTR) (Zar 1999). Overall mean values (n = 9; 3 study areas × 3 years) were calculated for total abundance, species richness and diversity, and for abundance of each of the individual mammal species. Duncan’s multiple range test (DMRT), adjusted for multiple contrasts, was used to compare mean values based on ANOVA results. In all cases, the level of significance was at least P = 0.05.
3 Results
3.1 GTR and woody debris treatments
Mean diameters and heights of residual Pseudotsuga menziesii trees in GTR units ranged from 46–48 cm and 30–31 m, respectively (Table 1). In the forest sites, three additional tree species included Pinus contorta, A. lasiocarpa, and Picea spp. as well as Pseudotsuga menziesii with a range of diameters from 25 to 38 cm and heights from 24 to 30 m. The mean number of residual trees in GTR units ranged from 5–15/ha with a basal area of < 5 m2/ha; and was 340 overstory (≥ 20 m height) trees/ha in the uncut forest. Area of treatment sites was similar (Table 1). For woody debris, total volume and volume/ha were significantly (P ≤ 0.02) lower in the dispersed treatments than in windrows (Table 1). Mean length of windrow and volume of debris per meter of length were similar (P > 0.05) between windrow treatments, as were mean height and width of windrows.
| Table 1. Mean (n = 3 study areas) ± SE diameter (cm), height (m), and stand density of overstory (≥ 20 m height) coniferous trees, measurements of woody debris treatments, and results of analyses. Mean values followed by different letters are significantly different by DMRT. | |||||||
| Parameter and species | DISP | DISP+GTR | WINDR | WINDR+GTR | FOREST | Analysis | |
| Overstory conifers | |||||||
| Mean diameter | |||||||
| Pseudotsuga menziesii | - | 48.2 ± 1.9 | - | 46.3 ± 4.3 | 38.1 ± 3.5 | - | - |
| Mean height | |||||||
| Pseudotsuga menziesii | - | 30.9 ± 1.0 | - | 29.6 ± 1.2 | 29.2 ± 2.4 | - | - |
| Trees/ha | - | 5–15 | - | 5–15 | 340 ± 87 | - | - |
| F3,6 | P | ||||||
| Area (ha) | 4.47 ± 0.23 | 4.63 ± 0.03 | 5.83 ± 1.34 | 5.77 ± 1.38 | 100+ | 0.92 | 0.49 |
| Woody debris | |||||||
| Total volume (m3) | 735b ± 106 | 1084b ± 257 | 2504a ± 483 | 2996a ± 296 | - | 21.76 | <0.01 |
| Volume/ha (m3) | 167.9b ± 33.8 | 234.4b ± 56.5 | 504.1a ± 165.3 | 589.3a ± 148.3 | - | 6.81 | 0.02 |
| F1,2 | P | ||||||
| Windrow length (m) | - | - | 256.1 ± 22.6 | 411.7 ± 77.8 | - | 6.85 | 0.12 |
| Volume (m3) per m of windrow | - | - | 10.23 ± 2.62 | 8.06 ± 2.14 | - | 1.18 | 0.39 |
| Windrow height (m) | - | - | 2.26 ± 0.34 | 2.01 ± 0.35 | - | 6.81 | 0.12 |
| Windrow width (m) | - | - | 8.03 ± 0.97 | 7.32 ± 0.83 | - | 0.43 | 0.58 |
| DISP = Dispersed debris; DISP+GTR = Dispersed debris and green-tree retention; WINDR = Windrow of debris; WINDR+GTR = Windrow of debris and green-tree retention. | |||||||
3.2 Small mammal communities
A total of nine species of forest-floor small mammals, composed of 1553 individuals, were captured. M. gapperi was the most common species captured with 599 individuals, followed by P. maniculatus (274), N. amoenus (201), M. longicaudus (171), S. monticolus (157), S. cinereus (63), P. intermedius (38), M. pennsylvanicus (35), and M. erminea (15). Mean total abundance of small mammals per index-line was significantly different among treatment sites at Munro (F4,20 = 17.05; P < 0.01) and Kathleen (F4,20 = 23.99; P < 0.01), but not at Blaeberry (Table 2 and Fig. 2). At Munro, mean total abundance was highest (DMRT; P = 0.05) in the dispersed+GTR site at 26 animals per line, followed by the windrow, forest, and windrow-GTR sites (Table 2). At Kathleen, mean total abundance was highest (DMRT; P = 0.05) in the windrow and windrow-GTR sites at 24 animals per line. The other three sites ranged from 7–11 animals per line. The sites at Blaeberry tended to follow this latter pattern with 12 animals per line in the windrow, windrow+GTR, and forest sites, and 6–7 in the other two sites, but with generally lower numbers overall than the other two study areas. All study areas had significant (P ≤ 0.02) changes in total abundance with time (Table 2). There was a significant (F8,40 = 9.53; P < 0.01) treatment × time interaction at Munro owing to changes in number of animals in the windrow and windrow+GTR sites (Fig. 2a).
| Table 2. Mean ± SE (n = 3 or 5 samples) total abundance, species richness, and diversity of small mammals during 2010–2012 and results of RM-ANOVA for each block (study area). Within a row, columns with different letters are significantly different by Duncan’s multiple range test (DMRT), adjusted for multiple contrasts. No data were found to be correlated among years. View in new window/tab. |
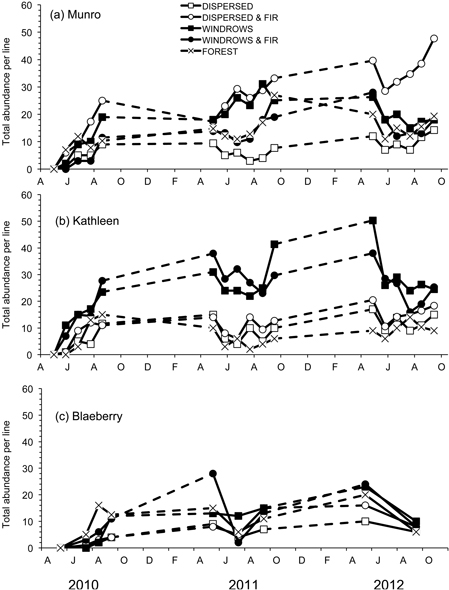
Fig. 2. Total abundance per line of forest-floor small mammals for the five treatments for each of the study sites: a) Munro, b) Kathleen, and c) Blaeberry 2010 to 2012. A = April, J = June, A = August, O = October, D = December, F = February.
Mean species richness was significantly (F4,20 = 3.58; P = 0.02) different among treatment sites at Munro, but not at Kathleen or Blaeberry (Table 2). Mean species richness was significantly (DMRT; P = 0.05) higher in the dispersed+GTR site than dispersed site, with the other sites being similar to both of these. Mean species diversity was similar (P > 0.05) among sites at all three study areas (Table 2). Both richness and diversity measures increased significantly (P < 0.01) with time at all study areas. Although not formally significant, the highest levels of species richness (4.39 and 4.28) and diversity (1.85 and 2.05) overall occurred in the windrow and windrow+GTR treatments, respectively, in 2012.
There was a positive relationship (r = 0.69; P = 0.01) between mean total abundance of small mammals and total volume of woody debris across the dispersed and windrow treatments with and without GTR (Fig. 3a). A similar relationship was recorded for mean species richness (r = 0.64; P = 0.03) with mean total volume of woody debris (Fig. 3b). Mean species diversity also followed this pattern but was not significant.
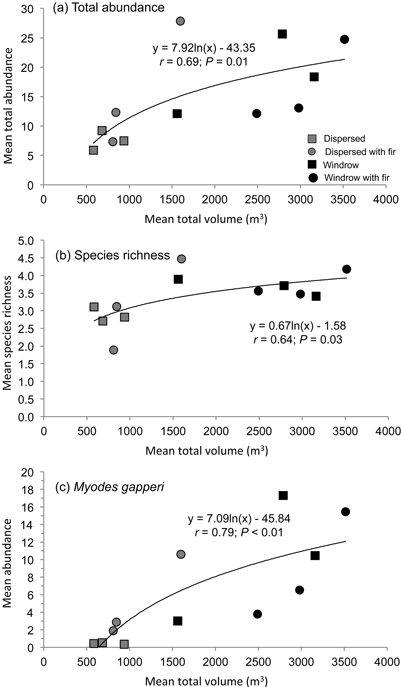
Fig. 3. Regression relationship of mean a) total abundance and b) species richness of forest-floor small mammals, and c) abundance of Myodes gapperi, with mean total volume of woody debris in dispersed and windrow treatments with and without GTR.
3.3 Red-backed voles
Susceptibility to capture was measured by Jolly trappability estimates with mean values ranging from 71.4 to 88.4% for M. gapperi in the sites where this species was common. Mean abundance of M. gapperi was significantly different among treatment sites at Munro (F4,20 = 83.08; P < 0.01) and Kathleen (F4,20 = 114.20; P < 0.01), but not at Blaeberry (Table 3 and Fig. 4). At Munro, mean abundance of M. gapperi was highest (DMRT; P = 0.05) in the dispersed+GTR and windrow sites at 10 voles per line, and at Kathleen was highest in the windrow and windrow+GTR sites at 16 voles per line (Table 3). Again, mean numbers of M. gapperi were generally lower at Blaeberry than at Munro or Kathleen, but with a relative increase in voles across sites with greater retention (Table 3). Munro and Kathleen had significant (P < 0.01) changes in abundance of M. gapperi with time, and all three study areas had significant (P ≤ 0.03) treatment × time interactions (Table 3 and Fig. 4).
| Table 3. Mean ± SE (n = 3 or 5 samples) abundance per line, number of recruits, successful pregnancies, and early juvenile survival for Myodes gapperi during 2010–2012 and results of RM-ANOVA for each block (study area). F-values identified by * were calculated using an H-F correction factor, which decreased the stated degrees of freedom due to correlation among repeated measures. Within a row, columns of mean values with different letters are significantly different by Duncan’s multiple range test (DMRT), adjusted for multiple contrasts. View in new window/tab. |
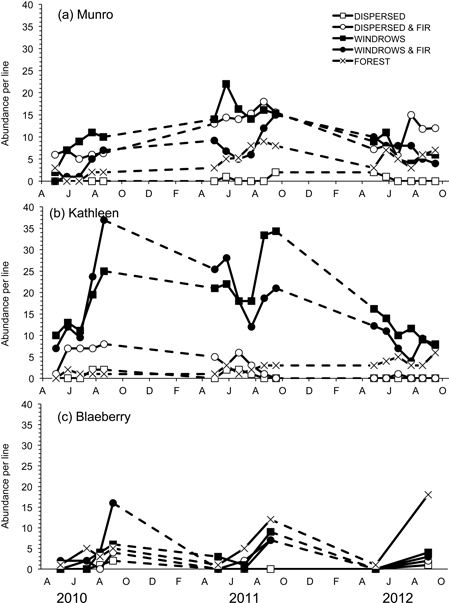
Fig. 4. Abundance per line of Myodes gapperi for the five treatments for each of the study sites: a) Munro, b) Kathleen, and c) Blaeberry 2010 to 2012. A = April, J = June, A = August, O = October, D = December, F = February.
Mean number of recruits also followed this pattern of abundance, with significant differences among treatment sites at Munro (F4,20 = 10.40; P < 0.01) and Kathleen (F4,20 = 17.25; P < 0.01) (Table 3). At Munro, mean number of recruits was highest (DMRT; P = 0.05) in the dispersed+GTR, windrow, and windrow+GTR sites, with the GTR sites being similar to the forest. At Kathleen, mean number of recruits was highest (DMRT; P = 0.05) in the windrow and windrow+GTR sites, followed by the dispersed+GTR and forest sites. At both of these study areas, the lowest number of recruits of M. gapperi was in the dispersed sites. At Blaeberry, recruit numbers were less than the other areas, but tended to increase with retention (Table 3). Munro and Kathleen had significant (P < 0.01) changes in mean number of recruits with time. Mean number of successful pregnancies and early juvenile survival followed the pattern of recruitment at Munro and Kathleen (Table 3). There were insufficient data to analyze pregnancies or juvenile survival for M. gapperi at Blaeberry, and similarly too few data for juvenile survival to be analyzed at Munro and Kathleen. There was a positive relationship (r = 0.79; P < 0.01) between mean abundance of M. gapperi and total volume of woody debris across treatments (Fig. 3c).
4 Discussion
4.1 GTR, woody debris and small mammals
This study is the first investigation of the responses of forest-floor small mammals to an increasing gradient of retained habitat structure via GTR and woody debris on clearcuts. Hypothesis (1), that abundance and species diversity of the forest-floor small mammal community would increase with higher levels of structural retention via GTR and woody debris seemed to be partly supported. Mean total abundance was higher in the two windrow treatment sites than the dispersed sites and did generally increase with the gradient of retained habitat structure. An exception was the dispersed+GTR site at Munro which had the highest number of small mammals at that study area. This result was likely owing to a relatively high volume (ca. twice as much) of dispersed woody debris for that particular treatment site. Although mean species richness and diversity were generally similar among treatment sites, the highest levels of species richness and diversity were recorded in windrow and windrow+GTR treatments in 2012, as these measurements increased significantly during the 3-year study. In addition, all nine species of small mammals were present in the various treatments in terms of overall mean abundance (Table 4).
| Table 4. Responses (overall mean values ± SE, n = 9; 3 study areas × 3 years) of small mammal communities to GTR and woody debris treatments 2010–2012. | |||||
| Parameter | Dispersed | Dispersed+GTR | Windrow | Windrow+GTR | Uncut forest |
| Mean abundance per line | |||||
| Myodes gapperi | 0.43 ± 0.12 | 5.08 ± 1.61 | 10.15 ± 2.50 | 8.40 ± 2.23 | 4.35 ± 0.91 |
| Peromyscus maniculatus | 2.07 ± 0.55 | 3.80 ± 0.76 | 1.55 ± 0.74 | 1.80 ± 0.83 | 3.95 ± 0.91 |
| Neotamias amoenus | 3.03 ± 0.87 | 4.89 ± 1.62 | 2.34 ± 0.71 | 2.28 ± 0.73 | 1.68 ± 0.76 |
| Phenacomys intermedius | 0.34 ± 0.11 | 0.02 ± 0.02 | 0.15 ± 0.11 | 0.36 ± 0.26 | 0.16 ± 0.07 |
| Microtus longicaudus | 0.53 ± 0.28 | 0.80 ± 0.61 | 2.61 ± 0.82 | 1.97 ± 0.65 | 0.74 ± 0.50 |
| Microtus pennsylvanicus | 0.45 ± 0.33 | 0.56 ± 0.27 | 0.00 ± 0.00 | 0.07 ± 0.07 | 0.06 ± 0.06 |
| Sorex monticolus | 0.58 ± 0.16 | 0.32 ± 0.17 | 1.45 ± 0.22 | 0.79 ± 0.19 | 0.29 ± 0.14 |
| Sorex cinereus | 0.14 ± 0.05 | 0.15 ± 0.09 | 0.51 ± 0.20 | 0.57 ± 0.20 | 0.26 ± 0.15 |
| Mustela erminea | 0.04 ± 0.02 | 0.04 ± 0.04 | 0.05 ± 0.04 | 0.16 ± 0.07 | 0.04 ± 0.03 |
| Total | 7.61 ± 0.74 | 15.66 ± 3.58 | 18.81 ± 2.50 | 16.40 ± 2.38 | 11.53 ± 1.23 |
| Species richness | 2.89 ± 0.15 | 3.14 ± 0.43 | 3.70 ± 0.30 | 3.72 ± 0.21 | 3.01 ± 0.27 |
| Species diversity | 1.16 ± 0.07 | 1.19 ± 0.16 | 1.39 ± 0.16 | 1.51 ± 0.17 | 1.29 ± 0.13 |
This pattern of higher values for the small mammal community was also reported for substantial piles and windrows of debris than dispersed debris by Sullivan et al. (2012). However, increasing levels of GTR, without woody debris structures, had no effect on abundance, richness, or diversity for the small mammal community in mixed Pinus contorta – Pseudotsuga menziesii forest similar to this study at three years (Sullivan and Sullivan 2001) and eight years (Sullivan et al. 2008) post-harvest. At 4-to 6-years post-harvest in this same forest type, even relatively high densities (73–127 trees/ha) and basal area (mean of 14.7 m2/ha) of residual trees, without woody debris, showed similar levels of total abundance and diversity in GTR and clearcut sites (Sullivan and Sullivan 2011). Much of this lack of positive responses to GTR is related to the species-specific responses of small mammals to harvesting strategies with habitat generalist and early successional species dominating clearcuts and closed-canopy specialists like M. gapperi disappearing (Fisher and Wilkinson 2005; Zwolak 2009).
4.2 Red-backed voles
Hypothesis (2), that abundance, reproduction, and recruitment of M. gapperi would increase with higher levels of structural retention via GTR and woody debris, seemed to be supported. Mean abundance, recruitment, and number of successful pregnancies of M. gapperi increased significantly with GTR and amounts of woody debris in windrows. In terms of GTR, M. gapperi has persisted for 2–3 years in several forest types that have been harvested by selection, patch, and shelterwood systems (Steventon et al. 1998; Von Trebra et al. 1998), but not at 3–8 years post-harvest in seed-tree systems (Sullivan et al. 2008). However, several other studies reported that red-backed voles commonly inhabit older clearcuts that did not have intensive site preparation or conifer release treatments (Gagne et al. 1999; Fuller et al. 2004; Ransome et al. 2009). M. gapperi habitat seemed ameliorated further when these parameters were augmented by advanced coniferous regeneration (Potvin et al. 1999), substantial woody debris on harvest openings (Sullivan et al. 2012) and in partially cut stands (Fauteux et al. 2012), and variable levels of both dispersed and aggregated GTR (Gitzen et al. 2007).
4.3 Diversifying clearcuts
Hypothesis (3), that responses of the forest-floor small mammal community and M. gapperi, on sites with GTR and windrows of debris, would be comparable to or higher than those in uncut mature/old growth forest was also supported, at least for this initial 3-year post-harvest window of investigation. The presence of M. gapperi populations at mature or old-growth “forest” levels of abundance suggested that networks of food sources and predators may also have been present as components of biodiversity, however, we did not collect data on these aspects. Abundances of mammalian carnivores, and hence degree of predation, are also reduced by clearcutting with loss of preferred prey species, den sites, and other components of forest stand structure (Fisher and Wilkinson 2005). In terms of mammalian biodiversity, carnivores such as coyotes (Canis latrans Say), red foxes (Vulpes vulpes L.), lynx (Lynx canadensis Kerr), cougars (Felis concolor L.), weasels, and American martens also use woody debris, particularly logs, as habitat for denning, nesting, and foraging (McComb 2003). Weasels and marten seem to use incidental debris piles in reports reviewed by Bunnell and Houde (2010). Thus, strategic management of post-harvest woody debris could help maintain abundance and diversity of forest mammals, both predator and prey species, on clearcuts. Large-scale salvage harvesting of those forests influenced by wildfire and insect outbreaks typically create large (> 100 ha) openings where habitat creation is much needed (Lindenmayer et al. 2008).
4.4 Study design
The three study areas were originally to act as regional replicates in a randomized complete block design. However, a significant treatment by block interaction necessitated analysis of each block (study area) separately. As noted, small mammals at the Munro and Kathleen study areas exhibited reasonably similar patterns of responses to the different treatments. Mammal responses to treatments at the Blaeberry study area tended to follow the same pattern but small sample sizes may have limited the detection of significant differences. Thus, inferences of results from these study areas should be cautiously extrapolated across the different forest ecological zones. However, it is important to note that these inferences reflect small mammal responses to habitat structures during summer and fall (May to October) only. Population changes resulting from these treatments may not have been the same during other seasons of the year. The 3-year study duration did suggest that there were no dramatic changes in abundance of small mammals from one year to the next during the overwinter periods when data were not available. As with many ecological studies, additional replicates and years of study may have improved the precision of our estimates and duration of observed results, but were not possible due to funding and logistical constraints.
4.5 Conclusions
There are two methods of intervention, at the time of harvest, to produce “old-forest” habitat and diversify clearcuts for mammals: 1) green-tree retention and 2) strategic management of post-harvest woody debris. Each method has been tried in certain situations, but our assessment of a combination of these two interventions suggested a potentially strong additive effect that appeared to be common to the three forest ecological zones examined (Fig. 5). The potential for both of these forest practices to be implemented in innovative strategies, to maintain or enhance habitat for forest mammals, is well within the scope of current industrial operations. However, planting of seedlings to regenerate cutover areas needs to avoid the perimeter of windrows where voles may feed on trees immediately adjacent to woody debris (Sullivan and Sullivan 2014). In the absence of woody debris as retained structures on new clearcuts, density and basal area of residual trees need to be increased substantially beyond the common levels examined in our study. Levels of GTR at ≥ 25 m2/ha basal area and ≥ 30% uncut forest seem to be GTR targets to maintain some aspects of habitat for forest mammals (Klenner and Sullivan 2009). In addition, the risk of windthrow of GTR structures also needs to be considered (Scott and Mitchell 2005; Lavoie et al. 2012). However, economic constraints suggest that clearcutting may continue as the dominant harvest system in temperate and boreal forests with relatively low amounts of GTR. Hence, woody debris structures should help provide some habitat to conserve forest mammals on harvest openings.
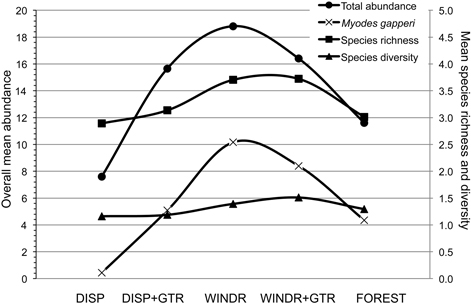
Fig. 5. Overall mean (n = 9; 3 study areas × 3 years) abundance for total small mammals and Myodes gapperi and species richness and diversity for the five treatments 2010 to 2012. DISP = Dispersed, DISP+GTR = Dispersed with green tree retention, WINDR = Windrow, WINDR+GTR = Windrow with green tree retention.
Acknowledgements
We thank Gorman Bros. Lumber Ltd., Louisiana-Pacific Corporation, and the Applied Mammal Research Institute for financial and logistical support. K. Rouck, S. Clerke, and S. King were particularly helpful in assisting with this project. We thank H. Sullivan for assistance with the fieldwork.
References
Bunnell F.L., Dunsworth G.B. (2009). Forestry and biodiversity: learning how to sustain biodiversity in managed forests. UBC Press, Vancouver, BC. 374 p.
Bunnell F.L., Houde I. (2010). Down wood and biodiversity – implications to forest practices. Environmental Reviews 18: 397–421. http://dx.doi.org/10.1139/A10-019.
Carey A.B., Harrington C.A. (2001). Small mammals in young forests: implications for management for sustainability. Forest Ecology and Management 154: 289–309. http://dx.doi.org/10.1016/S0378-1127(00)00638-1.
Carey A.B., Kershner J., Biswell B., DeToledo L.D. (1999). Ecological scale and forest development: squirrels, dietary fungi, and vascular plants in managed and unmanaged forests. Wildlife Monographs 142. The Wildlife Society, Bethesda, MD.
Ecke F., Lofgren O., Hornfeldt B., Eklund U., Ericsson P., Sorlin D. (2001). Abundance and diversity of small mammals in relation to structural habitat factors. Ecological Bulletins 49: 165–171.
Ecke F., Lofgren O., Sorlin D. (2002). Population dynamics of small mammals in relation to forest age and structural habitat factors in northern Sweden. Journal of Applied Ecology 39: 781–792. http://dx.doi.org/10.1046/j.1365-2664.2002.00759.x.
Fauteux D., Imbeau L., Drapeau P., Mazerolle M.J. (2012). Small mammal responses to coarse woody debris distribution at different spatial scales in managed and unmanaged boreal forests. Forest Ecology and Management 266: 194–205. http://dx.doi.org/10.1016/j.foreco.2011.11.020.
Fisher J.T., Wilkinson L. (2005). The response of mammals to forest fire and timber harvest in the North American boreal forest. Mammal Review 35: 51–81. http://dx.doi.org/10.1111/j.1365-2907.2005.00053.x.
Fowler J., Cohen L., Jarvis P. (1998). Practical statistics for field biology. 2nd ed. John Wiley & Sons. 259 p.
Franklin J.F., Berb D.R., Thornburgh D.A., Tappeiner J.C. (1997). Alternative silvicultural approaches to timber harvesting: variable retention harvest systems. In: Kohm K.A., Franklin J.F. (eds.). Creating a forestry for the 21st century: the science of ecosystem management. Island Press, Washington, DC. p. 111–139.
Franklin J.F., Lindenmayer D., MacMahon J.A., McKee A., Magnuson J., Perry D.A., Waide R., Foster D. (2000). Threads of continuity: ecosystem disturbances, biological legacies and ecosystem recovery. Conservation Biology in Practice 1: 8–16. http://dx.doi.org/10.1111/j.1526-4629.2000.tb00155.x.
Franklin J.F., Spies T.A., Van Pelt R., Carey A.B., Thornburgh D.A., et al. (2002). Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. Forest Ecology and Management 155: 399–423. http://dx.doi.org/10.1016/S0378-1127(01)00575-8.
Fuller A.K., Harrison D.J., Lachowski H.J. (2004). Stand scale effects of partial harvesting and clearcutting on small mammals and forest structure. Forest Ecology and Management 191: 373–386. http://dx.doi.org/10.1016/j.foreco.2004.01.014.
Gagne N., Bélanger L., Huot J. (1999). Comparative responses of small mammals, vegetation, and food sources to natural regeneration and conifer release treatments in boreal balsam fir stands of Quebec. Canadian Journal of Forest Research 29: 1128–1140. http://dx.doi.org/10.1139/x99-095.
Gitzen R.A., West S.D., Maguire C.C., Manning T., Halpern C.B. (2007). Response of terrestrial small mammals to varying amounts and patterns of green-tree retention in Pacific Northwest forests. Forest Ecology and Management 251: 142–155. http://dx.doi.org/10.1016/j.foreco.2007.05.028.
Gunther P.M., Horn B.S., Babb G.D. (1983). Small mammal populations and food selection in relation to timber harvest practices in the western Cascade Mountains. Northwest Science 57: 32–44.
Hardy C.C. (1996). Guidelines for estimating volume, biomass, and smoke production for piled slash. USDA Forest Service, Pacific Northwest Research Station, General Technical Report PNW-GTR-364. 17 p.
Harmon M.E., Franklin J.F., Swanson F.J. et al. (1986). Ecology of coarse woody debris in temperate ecosystems. Advances in Ecological Research 15: 133–302. http://dx.doi.org/10.1016/S0065-2504(08)60121-X.
Hurlbert S.H. (1984). Pseudoreplication and the design of ecological field experiments. Ecological Monographs 54: 187–211. http://dx.doi.org/10.2307/1942661.
Huynh H., Feldt L.S. (1976). Estimation of the Box correction for degrees of freedom from sample data in the randomized block and split-plot designs. Journal of Educational and Behavioral Statistics 1: 69–82. http://dx.doi.org/10.3102/10769986001001069.
Klenner W., Sullivan T.P. (2009). Partial and clear-cut harvesting of dry Douglas-fir forests: implications for small mammal communities. Canadian Journal of Forest Research 33: 2282–2296.
Krebs C.J. (1999). Ecological methodology. Addison Wesley Longman, Inc. 624 p.
Krebs C.J., Boonstra R. (1984). Trappability estimates for mark-recapture data. Canadian Journal of Zoology 62: 2440–2444. http://dx.doi.org/10.1139/z84-360.
Krebs C.J., Keller B.L., Tamarin R.H. (1969). Microtus population biology: Demographic changes in fluctuating populations of M. ochrogaster and M. pennsylvanicus in southern Indiana. Ecology 50: 587–607. http://dx.doi.org/10.2307/1936248.
Kuehl R.C. (1994). Repeated measures designs. In: Statistical principles of research design and analysis. Duxbury Press, Belmont, California. p. 499–528.
Larsen J.B. (1995). Ecological stability of forests and sustainable silviculture. Forest Ecology and Management 73: 85–96. http://dx.doi.org/10.1016/0378-1127(94)03501-M.
Lavoie S., Ruel, J-C., Bergeron Y., Harvey B.D. (2012). Windthrow after group and dispersed tree retention in eastern Canada. Forest Ecology and Management 269: 158–167. http://dx.doi.org/10.1016/j.foreco.2011.12.018.
Lindenmayer D.B., Burton P.J., Franklin J.F. (2008). Salvage logging and its ecological consequences. Island Press, Washington, DC. 227 p.
Littel R.C. (1989). Statistical analysis of experiments with repeated measures. HortScience 24: 36–40.
Magurran A.E. (2004). Measuring biological diversity. Blackwell Publishing, Oxford, UK.
Martin S.K. (1994). Feeding ecology of American martens and fishers. In: Buskirk S.W., Harestad A.S., Raphael M.G., Powell R.A. (eds.). Martens, sables, and fishers. Biology and conservation. Comstock Publishing Associates, Cornell University Press, Ithaca and London. p. 297–315.
Maser C., Trappe J.M., Nussbaum R.A. (1978). Fungal-small mammal inter-relationships with emphasis on Oregon coniferous forests. Ecology 59: 799–809. http://dx.doi.org/10.2307/1938784.
McComb W.C. (2003). Ecology of coarse woody debris and its role as habitat for mammals. In: Zabel C.J., Anthony R.G. (eds.). Mammal community dynamics. Management and conservation in the coniferous forests of western North America. Cambridge University Press, Cambridge, UK. http://dx.doi.org/10.1017/CBO9780511615757.012.
McComb W.C., Lindenmayer D. (1999). Dying, dead, and down trees. In: Hunter M.L., Jr. (ed.). Maintaining biodiversity in forest ecosystems. Cambridge University Press, Cambridge, UK. http://dx.doi.org/10.1017/CBO9780511613029.012.
McComb W.C., Spies T.A., Emmingham W.H. (1993). Douglas-fir forests. Managing for timber and mature-forest habitat. Journal of Forestry 91: 31–42.
Meidinger D., Pojar J. (1991). Ecosystems of British Columbia. Research Branch, Ministry of Forests, Special Report Series 6.
Merritt J.F. (1981). Clethrionomys gapperi. Mammalian Species 146: 1–9. http://dx.doi.org/10.2307/3503900.
Nagorsen D.W. (1996). Opossums, shrews, and moles of British Columbia. Vol. 2. The mammals of British Columbia. UBC Press, Vancouver, BC, Canada.
Pearce J., Venier L. (2005). Small mammals as bioindicators of sustainable boreal forest management. Forest Ecology and Management 208: 153–175. http://dx.doi.org/10.1016/j.foreco.2004.11.024.
Pearson D.E., Ruggiero L.F. (2003). Transect versus grid trapping arrangements for sampling small-mammal communities. Wildlife Society Bulletin 31: 454–459.
Potvin F., Courtois R., Bélanger L. (1999). Short-term response of wildlife to clear-cutting in Quebec boreal forest: multiscale effects and management implications. Canadian Journal of Forest Research 29: 1120–1127. http://dx.doi.org/10.1139/x99-040.
Ransome D.B., Lindgren P.M.F., Waterhouse M.J., Armleder H.M., Sullivan T.P. (2009). Small mammal response to group-selection silvicultural systems in Engelmann spruce-subalpine fir forests: 14 years post harvest. Canadian Journal of Forest Research 39: 1698–1708. http://dx.doi.org/10.1139/X09-095.
Rosenvald R., Lohmus A. (2008). For what, when, and where is green-tree retention better than clear-cutting? A review of biodiversity aspects. Forest Ecology and Management 255: 1–15. http://dx.doi.org/10.1016/j.foreco.2007.09.016.
Scott R.E., Mitchell S.J. (2005). Empirical modelling of windthrow risk in partially harvested stands using tree, neighbourhood, and stand attributes. Forest Ecology and Management 218: 193–209. http://dx.doi.org/10.1016/j.foreco.2005.07.012.
Seber G.A.F. (1982). The estimation of animal abundance and related parameters. 2nd ed. Charles Griffin & Co. Ltd., London, England.
Smith D.M. (1986). The practice of silviculture. 8th ed. John Wiley and Sons, New York, NY.
Spies T.A., Franklin J.F., Thomas T.B. (1988). Coarse woody debris in Douglas-fir forests of western Oregon and Washington. Ecology 69: 1689–1702. http://dx.doi.org/10.2307/1941147.
SPSS. (2013). Institute Inc. Statistical Programs of the Social Sciences. Chicago, Illinois.
Steventon J.D., MacKenzie K.L., Mahon T.E. (1998). Response of small mammals and birds to partial cutting and clearcutting in northwest British Columbia. Forestry Chronicle 74: 703–713. http://dx.doi.org/10.5558/tfc74703-5.
Sullivan T.P., Sullivan D.S. (2001). Influence of variable retention harvests on forest ecosystems. II. Diversity and population dynamics of small mammals. Journal of Applied Ecology 38: 1234–1252. http://dx.doi.org/10.1046/j.0021-8901.2001.00674.x.
Sullivan T.P., Sullivan D.S. (2011). Balancing pest management and forest biodiversity: vole populations and habitat in clearcut vs. variable retention harvested sites. Crop Protection 30: 833–843. http://dx.doi.org/10.1016/j.cropro.2011.03.001.
Sullivan T.P., Sullivan D.S. (2014). Voles, trees, and woody debris structures as habitat: balancing forest crop protection and biodiversity. Crop Protection 60: 70–77. http://dx.doi.org/10.1016/j.cropro.2014.03.002.
Sullivan T.P., Sullivan D.S., Lindgren P.M.F. (2008). Influence of variable retention harvests on forest ecosystems: Plant and mammal responses up to 8 years post-harvest. Forest Ecology and Management 254: 239–254. http://dx.doi.org/10.1016/j.foreco.2007.08.005.
Sullivan T.P., Sullivan D.S., Lindgren P.M.F., Ransome D.B., Bull J.G., Ristea C. (2011). Bioenergy or biodiversity: woody debris structures and maintenance of red-backed voles on clearcuts. Biomass and Bioenergy 35: 4390–4398. http://dx.doi.org/10.1016/j.biombioe.2011.08.013.
Sullivan T.P., Sullivan D.S., Lindgren P.M.F., Ransome D.B. (2012). If we build habitat, will they come? Woody debris structures and conservation of forest mammals. Journal of Mammalogy 93: 1456–1468. http://dx.doi.org/10.1644/11-MAMM-A-250.1.
Tallmon D., Mills L.S. (1994). Use of logs within home ranges of California red-backed voles on a remnant of forest. Journal of Mammalogy 75: 97–101. http://dx.doi.org/10.2307/1382240.
Vanderwel M.C., Malcolm J.R., Casperson J.P., Newman M.A. (2010). Fine-scale habitat associations of red-backed voles in boreal mixedwood stands. Journal of Wildlife Management 74: 1492–1501. http://dx.doi.org/10.1111/j.1937-2817.2010.tb01276.x.
Van Wagner C.E. (1968). The line intersect method in forest fuel sampling. Forest Science 14: 20–26.
Von Treba C., Lavender D.P., Sullivan T.P. (1998). Relations of small mammal populations to even-aged shelterwood systems in sub-boreal spruce forest. Journal of Wildlife Management 62: 630–642. http://dx.doi.org/10.2307/3802339.
Zar J.H. (1999). Biostatistical analysis. Prentice-Hall, Inc., Englewood Cliffs, New Jersey, USA.
Zwolak R. (2009). A meta-analysis of the effects of wildfire, clearcutting, and partial harvest on the abundance of North American small mammals. Forest Ecology and Management 258: 539–545. http://dx.doi.org/10.1016/j.foreco.2009.05.033.
Total of 61 references

