Does the pine weevil (Hylobius abietis) prefer conifer seedlings over other main food sources?
Fedderwitz F., Björklund N., Ninkovic V., Nordlander G. (2018). Does the pine weevil (Hylobius abietis) prefer conifer seedlings over other main food sources? Silva Fennica vol. 52 no. 3 article id 9946. https://doi.org/10.14214/sf.9946
Highlights
- Adult pine weevils feed on seedlings and mature conifers, but cause economic damage only on seedlings
- Their feeding preferences for branches and roots over seedlings were tested in a laboratory experiment
- The only clear preference was for Norway spruce roots
- Results support new approaches of seedling protection attempting to redirect pine weevils from planted seedlings to other food sources.
Abstract
Adult pine weevils (Hylobius abietis (L.)) feed on the tender bark of branches and roots of mature conifer trees and on the stem bark of conifer seedlings. Their feeding on mature trees does not cause any economic damage, but their feeding on planted seedlings is so devastating that the pine weevil is considered one of the most important forest pest insects in Europe. We asked whether the pine weevil prefers seedlings over other regularly utilized food sources. This question is of particular interest because new approaches to seedling protection are based on decreasing any preference for seedlings by using less palatable plants or by enhancing their defence (by genetic selection or by methyl jasmonate treatment). In a laboratory choice experiment we tested pine weevil feeding preferences for seedlings compared with branches and roots from mature trees (separately for Norway spruce and Scots pine). Pine weevils preferred roots, but not branches, of Norway spruce over seedlings of the same species. With Scots pine there were no clear preferences, but the weevils showed a tendency to prefer roots over seedlings. These results provide support for seedling protection approaches that attempt to redirect pine feeding from planted seedlings to other food sources.
Keywords
Pinus sylvestris;
Picea abies;
forest regeneration;
feeding preference;
herbivore;
plant tissues
-
Fedderwitz,
Department of Ecology, Swedish University of Agricultural Sciences, Box 7044, SE-750 07 Uppsala, Sweden
E-mail
frauke.fedderwitz@slu.se

- Björklund, Department of Ecology, Swedish University of Agricultural Sciences, Box 7044, SE-750 07 Uppsala, Sweden E-mail niklas.bjorklund@slu.se
- Ninkovic, Department of Ecology, Swedish University of Agricultural Sciences, Box 7044, SE-750 07 Uppsala, Sweden E-mail velemir.ninkovic@slu.se
- Nordlander, Department of Ecology, Swedish University of Agricultural Sciences, Box 7044, SE-750 07 Uppsala, Sweden E-mail goran.nordlander@slu.se
Received 8 January 2018 Accepted 16 May 2018 Published 23 May 2018
Views 81382
Available at https://doi.org/10.14214/sf.9946 | Download PDF

1 Introduction
The pine weevil (Hylobius abietis (L.)) is one of the most important pest insects in European forests (Långström and Day 2004). It causes much economic damage by feeding on the bark of newly planted conifer seedlings (Eidmann 1974; Petersson and Örlander 2003; Day et al. 2004). This feeding damage was already recognized as a problem about two centuries ago by Ratzeburg (1839), who described three- to six-year old plants as the preferred food of the adult pine weevil. Although pine weevils also feed on roots and branches of mature conifer trees (Eidmann 1974; Örlander et al. 2000; Wallertz et al. 2006), economic damage is limited to their feeding on planted seedlings; a considerable amount of pine weevil research has therefore focused on their interactions with seedlings (e.g. Fedderwitz et al. 2016; Luoranen et al. 2017; Zas et al. 2017).
The pine weevil feeds on plant material with diameters ranging from about 2 mm to 20 mm, with an apparent preference for diameters around 10 mm – in seedlings as well as in branches or roots of mature trees (Örlander et al. 2000; Wallertz et al. 2005). Feeding in canopies occurs especially during early summer, just after the pine weevils complete their long distance migration flight (Örlander et al. 2000; Solbreck 1980). The crowns of conifers surrounding a clear-cut, to which migrating pine weevils are attracted, provide, in theory, enough food for the local pine weevil population (Escherich 1923; Örlander et al. 2000). Similarly, the roots of mature conifers left on a fresh clear-cut are a substantial food source for pine weevils (Wallertz et al. 2006). In addition, understory vegetation and fresh branches left on the ground are utilized by the pine weevil (Örlander et al. 2001). Fresh cut material is, in comparison to intact trees or seedlings, more vulnerable to attack as it cannot up-regulate its induced defences.
Nevertheless, pine weevils feed extensively on planted seedlings; which raises the question of whether seedlings are a preferred food source compared to the tissues of mature trees. This question becomes especially relevant as new seedling protection methods involve the development of better-defended seedlings by decreasing their palatability (Holopainen et al. 2009; Zas et al. 2014). For example, seedlings treated with methyl jasmonate – a naturally occurring plant hormone – are less likely to be fed on if other food sources are available (Zas et al. 2014; Fedderwitz et al. 2016). This protective effect, however, tends to disappear in a no-choice situation (Fedderwitz et al. 2016). Furthermore, the genetic variation in resistance may be explored to select the more resistant genotypes for planting (Zas et al. 2017). The efficiency of these methods is likely to be related to the difference in preference between seedlings and any alternative food resources.
In this study we investigated feeding preferences of the pine weevil with regard to different host plant tissues within species, i.e. the tender bark of branches and roots of mature trees compared to stems of seedlings. Such comparisons have not been conducted before, although feeding on seedlings and cuttings (i.e. plants propagated from stem cuttings) has been compared (Hannerz et al. 2002; Kennedy et al. 2006) and preferences among different tree species has been the subject of several studies (e.g. Leather et al. 1994; Manlove et al. 1997; Månsson and Schlyter 2004; Toivonen and Viiri 2006; Zas et al. 2011; Wallertz et al. 2014). Thus, we evaluate which of the food sources that adult pine weevils mainly use, are preferred in feeding experiments by comparing different tissues of the two most common conifers in the forests of northern Europe: Norway spruce (Picea abies (L.) Karst.) and Scots pine (Pinus sylvestris L.).
2 Materials and methods
2.1 Plant and insect material
Branches and roots were collected during early summer from five Scots pine and five Norway spruce trees (approximately 2.5 m high; in this comparison with seedlings regarded as “mature” trees) growing in the close vicinity of each other in a mixed forest stand 10 km south of Uppsala, Sweden. Used Norway spruce branches had an average diameter of 2.44 ± 0.12 mm and roots had an average diameter of 2.54 ± 0.08 mm (digital calliper (Biltema, Sweden)). Used Scots pine branches had an average diameter of 3.45 ± 0.08 mm and roots had an average diameter of 3.30 ± 0.16 mm. Branch and root samples were stored for one or two days in moist plastic bags at 10 °C until the start of the experiment. Containerized seedlings of Norway spruce (n = 19) and Scots pine (n = 25) were obtained from commercial nurseries where they had been produced for plantation in central Sweden. Norway spruce seedlings had an average stem diameter of 2.74 ± 0.08 mm (measured above the first/lowest branches) and an average height of about 20 cm. Scots pine seedlings had an average stem diameter of 3.50 ± 0.10 mm and an average height of about 15 cm.
Pine weevils were collected with traps on the ground on a clear-cut in the second year after harvest close to where the branch and root samples had been collected. Weevils were collected on the same day as the branch and root sampling, which was three days after the traps were set out.
2.2 Experimental procedure
We conducted two-choice feeding tests comparing stems of seedlings with branches and roots from mature trees. Comparisons were made within each of the two included tree species, Scots pine and Norway spruce.
Sixty male and 60 female pine weevils were placed individually in Petri dishes (14 cm in diameter) containing a water tube, but no food, on the day of collection. Sexes were equally assigned to the different tested pairs of food choices and tree species. With four combinations of mature material and tree species, each combination was thus tested with 15 male and 15 female weevils. The choice tests were conducted on two consecutive days due to practical reasons. Because all weevils were placed in the petri dishes simultaneously on the day of collection, some of the weevils were presented with food after 24 hours of starving and others after 48 hours of starving. For comparison, the same number of pine weevils was presented with each food choice each day, but not between days. The food was left with the weevils for 24 hours. Experiments were conducted at room temperature (22 °C).
Each seedling was cut above the first node and divided into 4 cm lengths until the recent year’s growth was reached. Each piece was paired with a 4 cm length of either a branch or root of the same species. Within each pair the difference in diameter was kept to a minimum (paired t-test; spruce: p = 0.86, pine: p = 0.24; n = 120; Microsoft Excel 2010, Version 14.0, Microsoft Corporation, USA). The phloem thickness for each piece was determined by cutting one slice of the end with a scalpel and measuring the thickness under a binocular microscope (Wild Heerbrugg) at 50 times magnification. Both ends of each piece were wrapped with aluminium foil leaving a 2 cm long opening in between. If present, needles were removed before wrapping.
The two pieces of a pair of the same tree species (branch-seedling or root-seedling) were placed at opposite sides of the Petri dish (Fig. 1). The water tube and one pine weevil were placed in the middle without being pointed at either piece. The water tube was not in contact with the wall of the Petri dish, allowing the weevil to walk along the wall of the Petri dish. After 24 hours (18L:6D) the area that had been eaten on each piece of host material was estimated in square millimetres using 1-mm grid graph paper as reference.
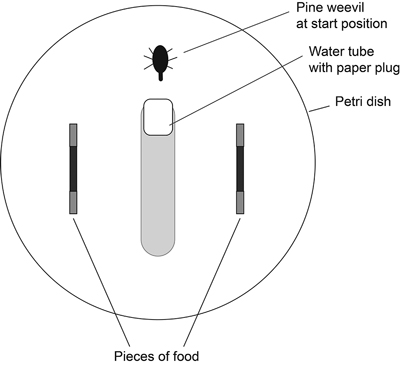
Fig. 1. Set-up of choice experiment. Two pieces of food of the same tree species (branch-seedling or root-seedling) were placed in each Petri dish. The ends of the food pieces were wrapped in aluminium foil. A water tube and one pine weevil were placed in the middle without being pointed at either piece.
2.3 Analyses
The amount of material that the pine weevils consumed was calculated from the area assessed as having been eaten and the phloem thickness. Pine weevils that did not feed at all were excluded from the analysis (in total 9 weevils). The relative preference of each pine weevil for the tissues from mature trees was calculated using the difference between the two food choices divided by the total amount eaten.
For the comparisons with Norway spruce roots, Scots pine branches and Scots pine roots, the preference was assessed with a one sample t-test testing against zero, which would mean no preference (Minitab version 16.1.0; Minitab, State College, PA, USA). For comparisons with Norway spruce branches a Wilcoxon Signed Rank Test was used since the data were not normally distributed (Minitab). Mann Whitney test was used to determine if there were differences in preferences between the rounds after 24 and 48 h of starvation or between female and male weevils. Kruskal-Wallis tests (Minitab) were performed to determine if there were differences in preference between the five individual trees from which the mature tree material was collected from.
3 Results
We found no differences in preferences between the rounds after 24 or 48 hours of starvation (spruce branches p = 0.93, spruce roots p = 0.69, pine branches p = 0.60, pine roots p = 0.95; Mann Whitney test), between female and male weevils (spruce branches p = 0.50, spruce roots p = 0.63, pine branches p = 0.45, pine roots p = 0.19; Mann Whitney test) or between mature tree individuals (spruce branches p = 0.43, spruce roots p = 0.26, pine branches p = 0.94, pine roots p = 0.65; Kruskal Wallis test), and therefore the data for both rounds, both sexes, and all tree individuals were analyzed together without specific regards for these factors.
Norway spruce roots were preferred over seedlings (p = 0.01; n = 29; one sample t-test; Fig. 2; Table 1) whereas there was no difference in preference between Norway spruce branches and seedlings (p = 0.12; n = 28; Wilcoxon Signed Rank Test). Similarly, there was a tendency of Scots pine roots being preferred over seedlings (p = 0.06; n = 29; one sample t-test) but no difference in preference between Scots pine branches and seedlings (p = 0.58; n = 25; one sample t-test).
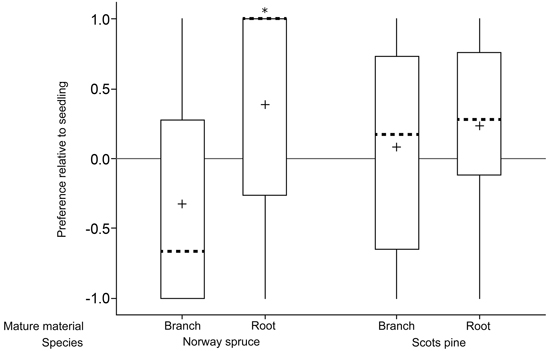
Fig. 2. Relative preferences for mature food sources (branch and root) compared to seedlings of the same species in a choice feeding test with individual pine weevils (+1 = preference for mature food source; 0 = no preference; –1 = preference for seedlings). Boxes include data from second and third percentiles with whiskers extending to the maximum/minimum data point within 1.5 box heights from the top/bottom of the box. Median line (dashed) and mean symbols (+) are shown. (* p < 0.05).
| Table 1. Amounts (mm3) of bark (phloem) consumed from mature food sources (branch and root) compared to seedlings of the same species presented to individual pine weevils in a 24 h choice feeding test. | |||||
| Species | Seedling compared with | N | Amount eaten mean ± SD (mm3) | Total amount eaten per tree species (mm3) | |
| Mature | Seedling | ||||
| Norway spruce | Branch | 28 | 5.79 ± 1.42 | 16.26 ± 3.08 | 1323 |
| Norway spruce | Root | 29 | 14.35 ± 2.17 | 9.99 ± 3.33 | |
| Scots pine | Branch | 25 | 14.09 ± 2.47 | 9.51 ± 1.63 | 1666 |
| Scots pine | Root | 29 | 24.73 ± 3.54 | 12.38 ± 2.28 | |
In the majority of the choice tests the pine weevils chose to feed more on roots than on seedlings, i.e. in 19 out of 29 and in 21 out of 29 cases, for Norway spruce and Scots pine, respectively (Fig. 3). There was no such clear pattern with regard to the amount fed on branches versus seedlings, i.e. in 8 out of 28 and 14 out of 25 cases they chose to feed more on branches, for Norway spruce and Scots pine, respectively.
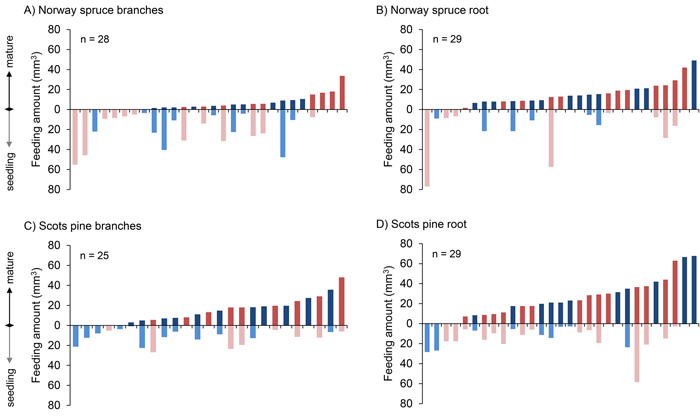
Fig. 3. Amount of bark (mm3) consumed by individual pine weevils in all four comparisons, ordered with respect to the increasing amounts of mature food (branch and root) consumed. Each bar represents an individual pine weevil (red: female, blue: male). View larger in new window/tab.
4 Discussion
Pine weevils fed on all food sources that were offered in the two-choice tests, i.e. branches and roots from mature trees and stems of seedlings of the two conifer species. That all these food sources are utilized is in agreement with previous field data (Örlander et al. 2000; Örlander et al. 2001; Wallertz et al. 2006). Interestingly, however, seedlings were not a preferred food source. On the contrary, pine weevils had a significant preference for roots over seedlings in Norway spruce, as well as a tendency toward such a preference in Scots pine.
The preference for roots might be due to the fact that roots that are not connected to a living tree can be used by pine weevils both for feeding and egg laying. Both sexes are attracted to and feed on the same substrate that is also used for oviposition (Nordlander et al. 1997; Bylund et al. 2004). In nature, the underground position of roots may further increase the incidence of feeding, since it has been shown that pine weevils generally prefer feeding in sheltered positions, for instance in the humus below ground surface (Nordlander et al. 2005). In our choice experiment, however, all food items were cut off and presented in a Petri dish, thus separated from any influence from their natural surroundings. Consequently, the preference for roots demonstrated here provides a conservative measure of the expected preference for roots in nature.
The nutritional value, in terms of nitrogen content, is higher in seedlings than in mature trees (Wainhouse et al. 2004; Iivonen et al. 2006). Wainhouse and coworkers (2004) pointed out that, in a no-choice situation, greater feeding damage to tissues with lower nitrogen content does not necessarily reflect a preference but rather the need for increased consumption in order to maintain an equivalent nutritional intake. In a choice experiment, however, the insect would be expected to prefer the more nutritious food (seedling), and feed less on the lower quality food (branch and root). Therefore the results of our choice experiment showing more feeding on roots than on seedlings should not be interpreted as compensatory feeding. Moreover, a previous field experiment has shown that freshly cut branches placed on the ground can relieve seedlings from pine weevil feeding (Örlander et al. 2001), which indicates that cut branches are not of inferior quality as food compared to living seedlings. Thus, branches are apparently as attractive as seedlings to pine weevils, despite a higher nitrogen content in seedlings.
The results of the current study reflects the weevils´ preference for excised host materials with limited ability to trigger induced defences, i.e. in this respect similar to the fresh branches and the roots of cut trees which constitutes the main food resource for pine weevils on a clear-cut (Bylund et al. 2004). Whether these preferences between the different host materials change if pine weevils feeding tests are conducted on living tissues remains to be evaluated.
The result that seedlings were not a preferred food source provides support for new approaches to seedling protection attempting to redirect pine weevil feeding from planted seedlings to other food sources that are regularly used by the pine weevil and available in large quantities. These approaches include the selection of more resistant material in breeding programmes (Zas et al. 2017) and to enhance the defence of seedlings by methyl jasmonate treatment in the nursery (Zas et al. 2014; Fedderwitz et al. 2016; Lundborg et al. 2016a; Lundborg et al. 2016b). Thus, planting regeneration areas with less palatable seedlings should result in less damage occurring to planted seedlings; instead, feeding may be redirected to alternative food sources, such as bark of branches and roots of mature conifer trees, where no economic harm is caused. From a practical forestry point of view such approaches provide environmentally friendly methods to protect planted seedlings against one of the most important pest insects in European forests.
Acknowledgements
This study was part of the Swedish Hylobius Research Program financed by the Swedish forestry sector. It received additional funding from the Swedish Foundation for Strategic Research (Grant RBb08-003) and the Swedish research council FORMAS (Grant 942-2015-70).
References
Bylund H., Nordlander G., Nordenhem H. (2004). Feeding and oviposition rates in the pine weevil Hylobius abietis (Coleoptera: Curculionidae). Bulletin of Entomological Research 94(4): 307–317. https://doi.org/10.1079/BER2004304.
Day K.R., Nordlander G., Kenis M., Halldórson G. (2004). General biology and life cycles of bark weevils. In: Lieutier F., Day K.R., Battisti A., Grégoire J.-C., Evans H.F. (eds.). Bark and wood boring insects in living trees in Europe, a synthesis. Springer, Dordrecht. p. 331–349. https://doi.org/10.1007/978-1-4020-2241-8_14.
Eidmann H.H. (1974). Hylobius abietis L., Großer Brauner Rüsselkäfer, Vol. 2. In: Schwenke W. (ed.). Die Forstschädlinge Europas. [Forest pest insects in Europe]. Paul Parey, Hamburg and Berlin, Germany. p. 277–293.
Escherich K. (1923). Die Forstinsekten Mitteleuropas. Paul Parey, Berlin, Germany.
Fedderwitz F., Nordlander G., Ninkovic V., Björklund N. (2016). Effects of jasmonate-induced resistance in conifer plants on the feeding behaviour of a bark-chewing insect, Hylobius abietis. Journal of Pest Science 89(1): 97–105. https://doi.org/10.1007/s10340-015-0684-9.
Hannerz M., Thorsén Å., Mattsson S., Weslien J. (2002). Pine weevil (Hylobius abietis) damage to cuttings and seedlings of Norway spruce. Forest Ecology and Management 160(1–3): 11–17. https://doi.org/10.1016/S0378-1127(01)00467-4.
Holopainen J.K., Heijari J., Nerg A.M., Vuorinen M., Kainulainen P. (2009). Potential for the use of exogenous chemical elicitors in disease and insect pest management of conifer seedling production. Open Forest Science Journal 2: 17–24. https://doi.org/10.2174/1874398600902010017.
Iivonen S., Kaakinen S., Jolkkonen A., Vapaavuori E., Linder S. (2006). Influence of long-term nutrient optimization on biomass, carbon, and nitrogen acquisition and allocation in Norway spruce. Canadian Journal of Forest Research 36(6): 1563–1571. https://doi.org/10.1139/x06-035.
Kennedy S., Cameron A., Thoss V., Wilson M. (2006). Role of monoterpenes in Hylobius abietis damage levels between cuttings and seedlings of Picea sitchensis. Scandinavian Journal of Forest Research 21(4): 340–344. https://doi.org/10.1080/02827580600792582.
Långström B., Day K.R. (2004) Damage, control and management of weevil pests, especially Hylobius abietis. In: Lieutier F., Day K.R., Battisti A., Grégoire J.-C., Evans H.F. (eds.). Bark and wood boring insects in living trees in Europe, a synthesis. Springer, Dordrecht, The Netherlands. p. 415–444. https://doi.org/10.1007/978-1-4020-2241-8_19.
Leather S.R., Ahmed S.I., Hogan L. (1994). Adult feeding preferences of the large pine weevil, Hylobius abietis (Coleoptera, Curculionidae). European Journal of Entomology 91: 385–389.
Lundborg L., Fedderwitz F., Björklund N., Nordlander G., Borg-Karlson A.-K. (2016a). Induced defenses change the chemical composition of pine seedlings and influence meal properties of the pine weevil Hylobius abietis. Phytochemistry 130: 99–105. https://doi.org/10.1016/j.phytochem.2016.06.002.
Lundborg L., Nordlander G., Björklund N., Nordenhem H., Borg-Karlson A.K. (2016b). Methyl jasmonate-induced monoterpenes in Scots pine and Norway spruce tissues affect pine weevil orientation. Journal of Chemical Ecology 42(12): 1237–1246. https://doi.org/10.1007/s10886-016-0790-z.
Luoranen J., Viiri H., Sianoja M., Poteri M., Lappi J. (2017). Predicting pine weevil risk: Effects of site, planting spot and seedling level factors on weevil feeding and mortality of Norway spruce seedlings. Forest Ecology and Management 389: 260–271. https://doi.org/10.1016/j.foreco.2017.01.006.
Manlove J.D., Styles J., Leather S.R. (1997). Feeding of the adults of the large pine weevil, Hylobius abietis (Coleoptera: Curculionidae). European Journal of Entomology 94: 153–156.
Månsson P.E., Schlyter F. (2004). Hylobius pine weevils adult host selection and antifeedants: feeding behaviour on host and non-host woody scandinavian plants. Agricultural and Forest Entomology 6(2): 165–171. https://doi.org/10.1111/j.1461-9563.2004.00217.x.
Nordlander G., Nordenhem H., Bylund H. (1997). Oviposition patterns of the pine weevil Hylobius abietis. Entomologia Experimentalis et Applicata 85: 1–9. https://doi.org/10.1046/j.1570-7458.1997.00229.x.
Nordlander G., Bylund H., Björklund N. (2005). Soil type and microtopography influencing feeding above and below ground by the pine weevil Hylobius abietis. Agricultural and Forest Entomology 7(2): 109–113. https://doi.org/10.1111/j.1461-9555.2005.00257.x.
Örlander G., Nordlander G., Wallertz K., Nordenhem H. (2000). Feeding in the crowns of Scots pine trees by the pine weevil Hylobius abietis. Scandinavian Journal of Forest Research 15(2): 194–201. https://doi.org/10.1080/028275800750015000.
Örlander G., Nordlander G., Wallertz K. (2001). Extra food supply decreases damage by the pine weevil Hylobius abietis. Scandinavian Journal of Forest Research 16(5): 450–454. https://doi.org/10.1080/02827580152632847.
Petersson M., Örlander G. (2003). Effectiveness of combinations of shelterwood, scarification, and feeding barriers to reduce pine weevil damage. Canadian Journal of Forest Research 33(1): 64–73. https://doi.org/10.1139/x02-156.
Ratzeburg J.T.C. (1839). Die Forst-Insecten oder Abbildung und Beschreibung der in den Wäldern Preußens und der Nachbarstaaten als schädlich oder nützlich bekannt gewordenen Insecten. Nicolai’sche Buchhandlung, Berlin, Germany.
Solbreck C. (1980). Dispersal distances of migrating pine weevils, Hylobius abietis, Coleoptera: Curculionidae. Entomologia Experimentalis et Applicata 28(2): 123–131. https://doi.org/10.1111/j.1570-7458.1980.tb02997.x.
Toivonen R., Viiri H. (2006). Adult large pine weevils Hylobius abietis feed on silver birch Betula pendula even in the presence of conifer seedlings. Agricultural and Forest Entomology 8(2): 121–128. https://doi.org/10.1111/j.1461-9563.2006.00290.x.
Wainhouse D., Boswell R., Ashburner R. (2004). Maturation feeding and reproductive development in adult pine weevil, Hylobius abietis (Coleoptera : Curculionidae). Bulletin of Entomological Research 94(1): 81–87. https://doi.org/10.1079/ber2003283.
Wallertz K., Örlander G., Luoranen J. (2005). Damage by pine weevil Hylobius abietis to conifer seedlings after shelterwood removal. Scandinavian Journal of Forest Research 20(5): 412–420. https://doi.org/10.1080/02827580500306954.
Wallertz K., Nordlander G., Örlander G. (2006). Feeding on roots in the humus layer by adult pine weevil, Hylobius abietis. Agricultural and Forest Entomology 8(4): 273–279. https://doi.org/10.1111/j.1461-9563.2006.00306.x.
Wallertz K., Nordenhem H., Nordlander G. (2014). Damage by the pine weevil Hylobius abietis to seedlings of two native and five introduced tree species in Sweden. Silva Fennica 48(4) article 1188. https://doi.org/10.14214/sf.1188.
Zas R., Moreira X., Sampedro L. (2011). Tolerance and induced resistance in a native and an exotic pine species: relevant traits for invasion ecology. Journal of Ecology 99(6): 1316–1326. https://doi.org/10.1111/j.1365-2745.2011.01872.x.
Zas R., Björklund N., Nordlander G., Cendan C., Hellqvist C., Sampedro L. (2014). Exploiting jasmonate-induced responses for field protection of conifer seedlings against a major forest pest, Hylobius abietis. Forest Ecology and Management 313: 212–223. https://doi.org/10.1016/j.foreco.2013.11.014.
Zas R., Björklund N., Sampedro L., Hellqvist C., Karlsson B., Jansson S., Nordlander G. (2017). Genetic variation in resistance of Norway spruce seedlings to damage by the pine weevil Hylobius abietis. Tree Genetics & Genomes 13: 111. https://doi.org/10.1007/s11295-017-1193-1.
Total of 31 references.

