Adaptive performance of genetically improved and unimproved seedlings of Scots pine
Haapanen M., Ruotsalainen S. (2021). Adaptive performance of genetically improved and unimproved seedlings of Scots pine. Silva Fennica vol. 55 no. 5 article id 10534. https://doi.org/10.14214/sf.10534
Highlights
- We studied the variation in adaptive traits in one-year-old seedlings of Scots pine representing different levels of genetic gain and geographical origins
- All the adaptive traits analyzed showed clinal co-variation with the latitude of origin
- Differences in adaptive performance between genetically improved and unimproved reproductive materials were mostly small and insignificant when the effect of the latitudinal origin was considered
- First-generation seed orchard materials implied slightly poorer autumn frost hardiness compared to other materials, but the results were ambiguous.
Abstract
Our main objective was to determine whether various genetically improved reproductive materials of Scots pine (Pinus sylvestris L.) differ in growth rhythm, autumn cold acclimation and resilience from unimproved materials. The study consisted of two successive indoor experiments with Scots pine seedlings representing four levels of genetic gain (unimproved natural stands, first-generation seed orchards, 1.5-generation seed orchards and seed orchards established with freezing-tested parents) and a wide range of geographical origins within Finland. The seedlings were assessed for terminal shoot elongation, growth cessation, bud set, freezing injuries and bud flushing over the first growth period. All the adaptive traits showed a latitudinal trend regardless of the genetic level. Seed orchard progenies and natural stand progenies did not differ significantly in the timing of growth cessation, bud set, and the flushing rate of the frost-injured seedlings, after the trait variation was adjusted to the latitude of origin. The differences in autumn frost hardiness were insignificant, too, except for the somewhat higher injury rate displayed by the first-generation seed orchard materials. The finding was not conclusive due to ambiguous results from the two experiments. Overall, we did not find evidence of alarming compromises in the adaptive performance of genetically improved materials.
Keywords
Pinus sylvestris;
cold hardiness;
growth rhythm;
seed orchards;
bud set;
freezing test;
reproductive materials
-
Haapanen,
Natural Resources Institute Finland (Luke), Production systems, Latokartanonkaari 9, FI-00790 Helsinki, Finland
 https://orcid.org/0000-0003-3294-501X
E-mail
matti.haapanen@luke.fi
https://orcid.org/0000-0003-3294-501X
E-mail
matti.haapanen@luke.fi

-
Ruotsalainen,
Natural Resources Institute Finland (Luke), Production systems, Vipusenkuja 5, FI-57200 Savonlinna, Finland
 https://orcid.org/0000-0002-2547-0282
E-mail
seppo.ruotsalainen@luke.fi
https://orcid.org/0000-0002-2547-0282
E-mail
seppo.ruotsalainen@luke.fi
Received 26 February 2021 Accepted 15 October 2021 Published 19 October 2021
Views 48929
Available at https://doi.org/10.14214/sf.10534 | Download PDF

1 Introduction
Scots pine (Pinus sylvestris L.) in Finland has been a subject of genetic improvement activities for more than 70 years. The first selection program was initiated in the late 1940’s and resulted in over 7000 plus trees selected for their outstanding growth and quality in natural stands (Oskarsson 1972). Grafted propagules of the plus trees were subsequently used to establish first-generation and 1.5-generation seed orchards which supply most of the reproductive materials used in Finnish forestry. As of 2020, over 90% of the 42 million nursery seedlings of Scots pine were raised from seed orchard seed. Orchard-reproduced materials are also extensively used in direct seeding, an equally popular regeneration method (to planting) of Scots pine in Finland (Natural Resources Institute Finland 2021).
The most advanced improved reproductive materials of Scots pine yield over 20% increment in per unit area wood production (Rosvall et al. 2001; Haapanen et al. 2016; Jansson et al. 2017). Gains in growth traits being undisputed, it is far less clear whether the selective breeding process has resulted in adverse changes in adaptively important traits through the system of genetic correlations. Some authors (Sarvas 1970; Mikola 1982; Andersson 1986; MacLachlan et al. 2017) have suggested that the phenotypic selection of plus trees, which were among the tallest trees of their stands, might have unintentionally favored trees with a prolonged growth period. This possibility has important implications since a long active period and especially a late bud set increase the risk of autumn frost injuries in the boreal climate (Mikola 1982).
If plus trees indeed manifested a longer-than-average active period, their progenies would likely be less hardy than the progenies of the unselected trees of the same origins and show performance reminiscent of northward transferred seedlots in common-garden trials (Berlin et al. 2016). Some studies lend cautious support to such an expectation. Andersson (1986) reported a slightly increased rate of freezing damages in seed orchard materials compared to wild trees. Andersson et al. (2007) found that the survival of controlled-crossed plus-tree progenies in northern Sweden was marginally lower (3.2% and 1.4% at ages 10.5 and 27.4 years, respectively) relative to wild stand material. Mikola (1993) presented results from a 10-year-old Scots pine field trial in northern Finland where the progenies of controlled-crossed northern plus trees showed at least 10% lower survival compared to the similar unimproved origin. On the other hand, Berlin et al. (2016) tested the transfer models developed for unimproved seed sources and found them to be valid for plus-tree progenies. Consequently, the recent deployment recommendations for seed orchards of Scots pine (Berlin et al. 2019) assume that the hardiness of plus-tree progenies is not different from unimproved trees.
Scots pine seedlots from 1.5-generation seed orchards are rapidly displacing first-generation orchard materials in Finnish nurseries (Natural Resources Institute Finland 2021). The 1.5-generation seed orchards comprise fewer (25 to 45) plus trees selected for their superior progeny-test or freezing-test performance (Haapanen et al. 2016) than the first-generation seed orchards which typically accommodate a much larger base (50 to 300) of plus trees. It seems plausible that the stricter selection would entail an opportunity both for genetic improvement and deterioration, depending on the sign and the magnitude of genetic correlations between selected traits and adaptive traits. Rehfeldt (1992), in a study with ponderosa pine (Pinus ponderosa Douglas ex C. Lawson), suggested that intense selection for increased growth would induce a correlated genetic change in growth rhythm toward populations from milder environments. Such changes would obviously have practical implications on the optimal deployment of the improved seeds and seedlings. Because of the wide commercial use of such materials in Finnish forestry, we consider it important to evaluate whether they deviate from the original unselected base population in traits of adaptive significance.
For this study, we selected an extensive sample of reproductive materials of Scots pine that represent different levels of genetic gain and a wide range of geographical origins within Finland. Our objective was to determine whether seedling progenies of unimproved and improved origins differ in adaptive traits related to growth rhythm, frost hardiness and resilience. We also wanted to find out whether the materials from different categories of seed orchards demonstrate divergent performances in any of these traits.
2 Materials and methods
2.1 Materials
The study consisted of two indoor experiments and series of freezing tests (called with acronyms EXP1 and EXP2) that were conducted in two research facilities of the Natural Resources Institute Finland during the winter of 2018–2019 and the summer of 2019. The research material comprised 54 wind-pollinated seedlots from 12 wild pine stands and 29 seed orchards. Five seed orchards were represented with multiple seedlots of different crop years. The improved materials were from eight first-generation seed orchards (SO1.0), sixteen 1.5-generation seed orchards (SO1.5) and five seed orchards comprising northern Finnish plus trees selected for their superior freezing-test hardiness (SO1.5FT) (Fig. 1). These three classes of seed orchard materials and the unimproved stand materials constitute the four levels hereon referred to as “genetic groups”.
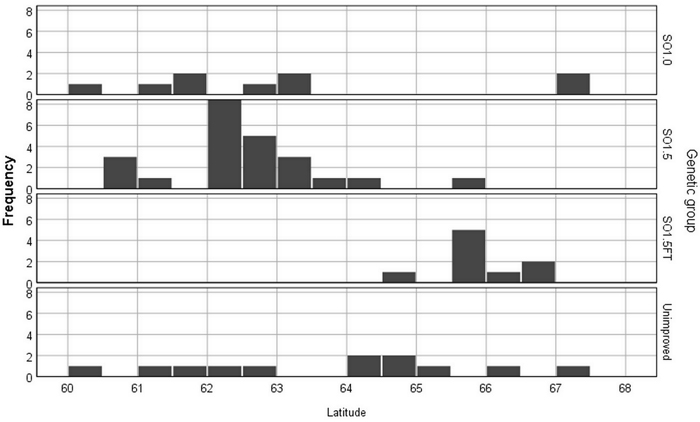
Fig. 1. The frequencies of the Pinus sylvestris seedlots by latitudinal origin and genetic group.
The seedlots originated from between 60°N and 68°N, which covers most of the latitude range of Finland. In the case of the unimproved seedlots, the origin was set equal to the latitude of the seed collection stand. For the orchard seedlots, the origin was determined based on the average latitude of the orchard clones (plus tree locations) and the latitude of the seed orchard location. The effect of the contaminating non-orchard pollen on the seedlot origin was estimated using the orchard-age dependent function described by Berlin et al. (2019).
The first experiment (EXP1) was sown in a climate-controlled greenhouse chamber in the Haapastensyrjä experimental unit (60°37´N, 24°26´E) at the end of November 2018. The study material comprised 46 seedlots and one hundred seedlings per seedlot (total 4600 seedlings). The sowing media consisted of one hundred peat filled PLANTEK 49F trays. Each tray with 49 cells (155 cm3) represented a complete block of the material (single-tree plots). During the first 10-week period, the seedlings were grown at 22/15 °C day/night temperatures and a 20-hour photoperiod. Physiological hardening measures were initiated in early February of 2019. The day/night temperatures were gradually reduced by 2 to 3 degrees until the target temperatures (8 °C day/3 °C night) were reached in early April of 2018. The photoperiod was simultaneously shortened from 20 to 8 hours in steps of 45 minutes. The terminal shoot elongation of 793 living seedlings in eighteen trays was assessed weekly between mid-January and early April of 2019, total twelve times or until growth cessation was observed.
Most of the materials (82 out of the 100 blocks) were subjected to freezing tests. Six tests were conducted at 2-to-5-day intervals between February 12 and April 4, 2019. The target temperature was –10 °C. The length of the cooling exposure was gradually prolonged from two to four hours to respond to the gradually improving cold acclimation of the seedlings. Two weeks after each freezing treatment, the primary needles of each seedling were visually assessed for the severity of injuries. A 7-class scoring was used to estimate to the proportion (0%, 10%, 30%, 50%, 70%, 90%, 100%) of discolored needle tissue. The analysis was performed on the percentage values treated as a continuous variable.
The second experiment (EXP2) was conducted in a plastic greenhouse in the Haapastensyrjä unit on May 28, 2019. The material comprised a total of 2450 seedlings from 38 seedlots of which 27 were common with the first experiment. The experimental design was like in EXP1 except for the number of replications (sowing trays) which consisted of 50 PLANTEK 49F trays. Eleven of the seedlots in EXP2 were replicated by sowing two seeds per tray to fill up all the tray cells. Ten trays (490 seedlings) were retained for the assessment of height growth which started on July 7, 2019 and was repeated weekly (13 times) until October 1, 2019. The remaining material was subjected to freezing in five batches between August 20 and September 9, 2019. The freezing tests were performed in the Haapastensyrjä experimental unit and in the Suonenjoki experimental unit (62°38´N, 27°03´E). The target temperatures varied from –10 °C to –15 °C.
In both experiments, the dates of germination and terminal bud formation (BUDSETDATE) were recorded on all the seedlings retained for the growth assessment. Finally, bud flushing (BUDFLUSH) was observed as a binary trait (0/1) on the seedlings subjected to freezing on June 24, 2019 (EXP1) and on June 17, 2020 (EXP2). The seedlings freezing-tested in EXP2 were stored outdoors over the winter of 2019/2020.
2.2 Analyses
A logistic growth model was fitted to the growth measurements of each normally developed (without secondary needles or other growth anomalies) seedling:
![]()
where H(t) is the height (mm) of the terminal shoot on the t:th day, TOTGR is the predicted total height increment, r is the logistic growth rate (steepness of the curve), t is the time passed since germination, and b is the time passed when the sigmoid function reaches its point of inflection. The parameters of the model were estimated using the procedure NLIN of the SAS statistical package (SAS Institute Inc 2013). The cessation of growth (GRCESS) was defined to occur when 95% of TOTGR was completed (Fig. 2).
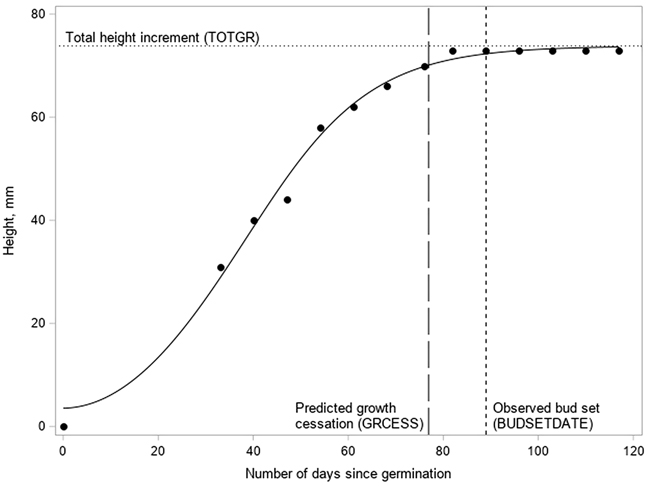
Fig. 2. The logistic growth curve fitted to the measurements (dots) of the terminal shoot elongation and the main phenological events in a Pinus sylvestris seedling.
The effects of the latitudinal origin and the genetic group on GRCESS and BUDSETDATE were estimated separately for the two experiments (Table 1), based on the following linear mixed model fit by the SAS/MIXED procedure (Littell et al. 2006):
![]()
where Yijk is the trait Y attributed to ijk:th seedling, Gi is the fixed effect of the i:th genetic group, Lj(i) is the latitudinal origin of the j:th seedlot (continuous) belonging to the i:th genetic group, β1 is the slope of overall regression, β2i is the slope of regression of the i:th genetic group, sj(i) is the random effect of the j:th seedlot within the i:th genetic group (sj(i) ~ iid (0, σ2s)), bk is the random effect of the k:th block (bk ~ iid (0, σ2b)) and eijk is the residual effect (eijk ~ iid (0, σ2e)). The model for freezing injuries (FRINJURY) followed Eq. 2 except for the inclusion of an additional random factor (freezing test, fl ~ iid (0, σ2f)).
| Table 1. Results from the mixed model analyses (the final models). The factors labelled as “Group”, “Origin” and “G × O” refer to the factors G, β1, β2i in Eq. 2. The factors denoted as “N/A” were not included in the final model. Significant differences (p < 0.05) are denoted in bold. The trait abbreviations are: GRCESS: Growth cessation; BUDSETDATE: Time from germination to the date when bud set was observed; FRINJURY: Freezing injury, the damaged needle area as percentage of the total needle area; BUDFLUSH: The percentage of freezing-tested seedlings with a recorded bud flush in June following the freezing. The predictions are for seedlings with 100% (EXP1) and 10% rates of needle injury (EXP2). | |||||||||
| Trait | Experiment | Fixed factors | Random factors | ||||||
| F-test (type 3) significance | Variance components | ||||||||
| Group | Origin | G × O | σ2f | σ2s | σ2b | σ2e | N | ||
| GRCESS | EXP1 | 0.057 | <0.000 | 0.044 | N/A | 0.1 | 1.1 | 25.8 | 594 |
| EXP2 | 0.178 | 0.133 | 0.150 | N/A | 4.8 | 19.5 | 80.3 | 445 | |
| BUDSETDATE | EXP1 | 0.070 | <0.000 | 0.057 | N/A | 0.0 | 10.6 | 91.7 | 524 |
| EXP2 | 0.089 | <0.000 | 0.069 | N/A | 2.3 | 8.9 | 58.3 | 444 | |
| FRINJURY | EXP1 | 0.317 | <0.001 | 0.315 | 468.5 | 8.5 | 154.2 | 650.4 | 3017 |
| EXP2 | 0.143 | <0.001 | 0.108 | 366.9 | 3.3 | 93.6 | 871.3 | 1290 | |
| FLUSHING | EXP1 | 0.738 | 0.085 | 0.731 | 1.3 | 0.0 | 0.9 | 3013 | |
| EXP2 | 0.272 | 0.099 | N/A | 0.0 | 0.0 | 3.8 | 1290 | ||
A generalized linear mixed model was fit to the binary data of BUDFLUSH using the SAS/GLIMMIX procedure (SAS Institute Inc 2017). The observations on flushing were modeled using a logit link function and a linear predictor:
![]()
where πijk is the probability of the bud-flushing event, η is the fixed intercept, Iijk is the injury percentage of the ijk:th seedling following freeze testing, and β denotes the slope of regression.
The least-square means for the three groups of seed orchards were compared against the similarly estimated mean for the unimproved materials at two latitudes, 62°N (southern Finland) and 66°N (northern Finland). The statistical significance of the pairwise differences was evaluated using the Dunnett’s post-hoc test (Littell et al. 2006). Tukey’s pos-hoc tests were also performed to analyze the variation among the three categories of seed orchards. No significant differences among the orchard groups were found for any of the traits. Because the results from Tukey’s tests were in a good agreement with those of Dunnett’s, we report only the results of the latter tests (Table 2).
| Table 2. Estimated least-square means and their 5% and 95% confidence limits (CLMlo and CLMhi) for the genetic groups in the two experiments and on the two latitudes representing southern (62°N) and northern (66°N) Finland. “Prob.” is the observed significance of Dunnett’s test for the difference between a seed orchard group vs. an unimproved control of the same origin. The pairwise differences found significant at the 5% alpha level are denoted in bold. SO1.0 seedlots of northern origin were not included in EXP1. Similarly, the SO1.5FT group represents exclusively northern origins and the results are shown only for the 66th latitude. The trait acronyms are given in Table 1. | ||||||||||
| Trait | Genetic group | Latitude | Experiment 1 | Experiment 2 | ||||||
| Mean | CLMlo | CLMhi | Prob. | Mean | CLMlo | CLMhi | Prob. | |||
| GRCESS (days) | Unimproved | 62 | 89.6 | 88.2 | 90.9 | 91.5 | 85.8 | 97.2 | ||
| SO1.0 | 62 | 89.5 | 88.2 | 90.7 | 0.991 | 87.4 | 82.8 | 91.0 | 0.249 | |
| SO1.5 | 62 | 89.9 | 89.0 | 90.8 | 0.857 | 86.9 | 82.8 | 91.0 | 0.165 | |
| Unimproved | 66 | 87.6 | 86.1 | 89.2 | 83.1 | 79.0 | 87.2 | |||
| SO1.0 | 66 | - | - | - | 77.4 | 72.7 | 82.2 | 0.063 | ||
| SO1.5 | 66 | 89.9 | 89.0 | 90.8 | 0.175 | 82.4 | 75.8 | 88.9 | 0.993 | |
| SO1.5FT | 66 | 89.6 | 88.5 | 90.7 | 0.183 | 81.5 | 77.5 | 85.4 | 0.779 | |
| BUDSETDATE (days) | Unimproved | 62 | 105.1 | 102.2 | 108.0 | 100.2 | 95.9 | 104.5 | ||
| SO1.0 | 62 | 106.6 | 103.9 | 109.3 | 0.414 | 97.5 | 94.3 | 100.7 | 0.527 | |
| SO1.5 | 62 | 105.4 | 103.5 | 107.4 | 0.859 | 95.6 | 92.7 | 98.4 | 0.104 | |
| Unimproved | 66 | 100.7 | 97.5 | 103.6 | 81.3 | 78.4 | 84.3 | |||
| SO1.0 | 66 | - | - | - | 84.9 | 81.3 | 88.4 | 0.175 | ||
| SO1.5 | 66 | 104.3 | 100.2 | 108.5 | 0.330 | 83.5 | 78.5 | 88.4 | 0.750 | |
| SO1.5FT | 66 | 102.7 | 100.3 | 105.1 | 0.537 | 81.7 | 78.8 | 84.5 | 0.994 | |
| FRINJURY (%) | Unimproved | 62 | 57.3 | 38.9 | 75.7 | 83.3 | 63.8 | 102.7 | ||
| SO1.0 | 62 | 64.2 | 45.8 | 85.5 | 0.016 | 84.0 | 65.1 | 103.0 | 0.995 | |
| SO1.5 | 62 | 61.7 | 43.6 | 79.9 | 0.082 | 83.1 | 64.4 | 101.8 | 0.999 | |
| Unimproved | 66 | 27.0 | 8.3 | 45.7 | 31.1 | 12.2 | 49.8 | |||
| SO1.0 | 66 | - | - | - | 43.3 | 24.2 | 62.5 | 0.071 | ||
| SO1.5 | 66 | 27.3 | 43.6 | 79.9 | 0.999 | 36.2 | 16.0 | 56.2 | 0.613 | |
| SO1.5FT | 66 | 23.8 | 8.3 | 45.7 | 0.578 | 28.1 | 9.4 | 46.8 | 0.688 | |
| BUDFLUSH (%) | Unimproved | 62 | 70.4 | 45.6 | 87.1 | 36.2 | 14.9 | 64.5 | ||
| SO1.0 | 62 | 71.4 | 47.0 | 87.5 | 0.991 | 35.0 | 12.0 | 68.1 | 0.999 | |
| SO1.5 | 62 | 66.7 | 42.3 | 84.6 | 0.676 | 33.6 | 14.4 | 60.4 | 0.986 | |
| Unimproved | 66 | 89.4 | 70.7 | 96.7 | 53.3 | 31.4 | 74.2 | |||
| SO1.0 | 66 | - | - | - | 52.2 | 27.6 | 75.8 | 0.999 | ||
| SO1.5 | 66 | 84.6 | 62.6 | 94.7 | 0.678 | 50.7 | 26.9 | 74.2 | 0.986 | |
| SO1.5FT | 66 | 91.5 | 77.6 | 97.1 | 0.891 | 58.0 | 36.2 | 77.0 | 0.903 | |
3 Results
The growth termination events GRCESS and BUDSETDATE were linearly related to the latitudinal origin: The northern origins ceased their growth before the southern ones regardless of the genetic level. Both events occurred considerably later in EXP1 compared to EXP2. Furthermore, the clinal trend was markedly less pronounced in EXP1 (Figs. 3 and 4). The southern SO1.5 materials in EXP2 appeared to have a slightly earlier BUDSETDATE than the unimproved materials (p < 0.104). However, none of the pairwise tests conducted in both experiments yielded a statistically significant result (Table 2).
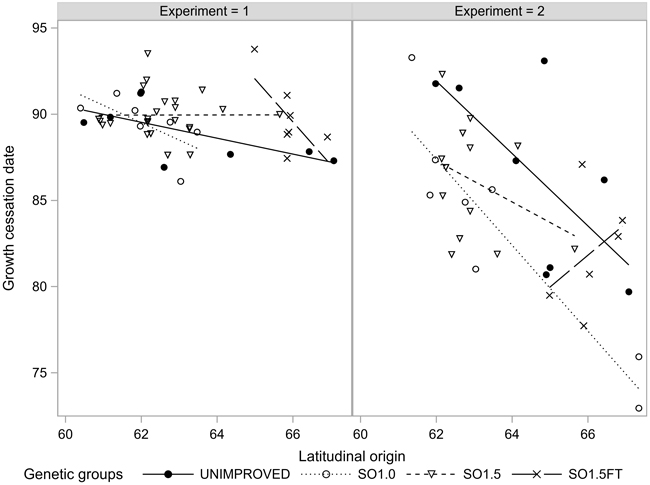
Fig. 3. Growth cessation (GRCESS) of the seedlots in the two experiments.
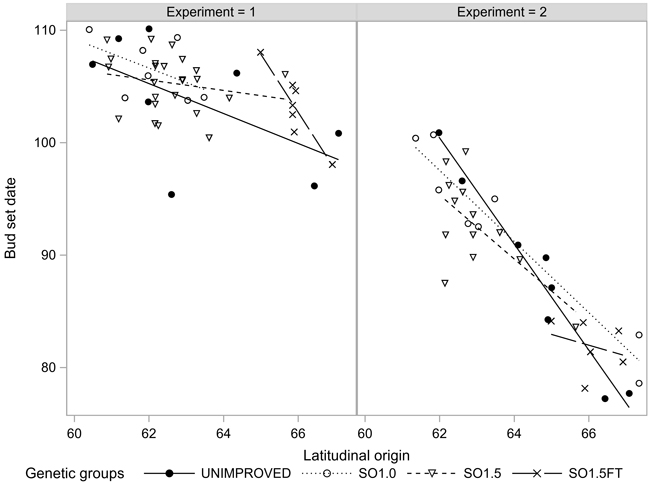
Fig. 4. Bud set (BUDSETDATE) of the seedlots in the two experiments.
The average injury rates were 51% and 54% in the freezing tests conducted in EXP1 (the range of individual tests was from 29 to 75%) and EXP2 (26 to 77%), respectively. The freezing injuries were closely related to the latitudinal origin of the seedlings (Fig. 5). The northern origins showed considerably (30% to 50%) milder injuries than the southern origins. The strong clinal pattern in the injury rates was also expressed in EXP1 which showed a weak latitudinal trend for the growth rhythm traits (Figs. 3–5).
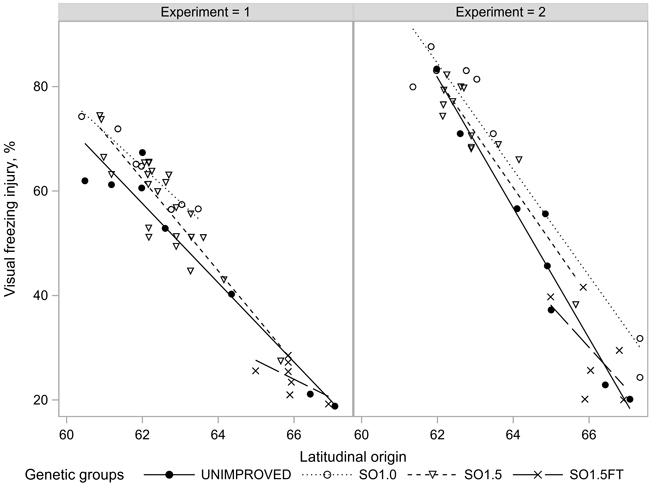
Fig. 5. Freezing injuries (FRINJURY) of the seedlots in the two experiments.
The differences in freezing injuries between the unimproved and improved progenies were mostly within a few percentage units and statistically non-significant with the single exception of the southern SO1.0 materials in EXP1, which were about 7% (p < 0.016) more injured compared to the unimproved materials. In EXP2, however, the genetic groups of southern origin differed in their injury rates less than one percentage unit (non-significant). On the 66th latitude, SO1.0 was, again, the most susceptible group, showing about 12% (p < 0.071) more injuries than the unimproved reference. The needle injuries of the SO1.5 materials were consistently between the unimproved and SO1.0 materials. SO1.5FT appeared as the hardiest genetic group in both experiments but, again, the post-hoc tests could not verify any of the differences as statistically significant (Table 2).
The proportion of the seedlings that flushed their buds in the June following the freezing test, varied from 22% (EXP2) to 84% (EXP1). Of the seedlings with at least 70% injured needles, 69% flushed in EXP1 and merely 2% in EXP2. The seedlings of the SO1.5FT group showed the highest flushing rates and the northern origins flushed at a slightly higher rate than the southern ones (Fig. 6). Still, no statistically significant differences in BUDFLUSH were found among the genetic groups and origins considering seedlings with the same severity of needle injury (Tables 1 and 2).
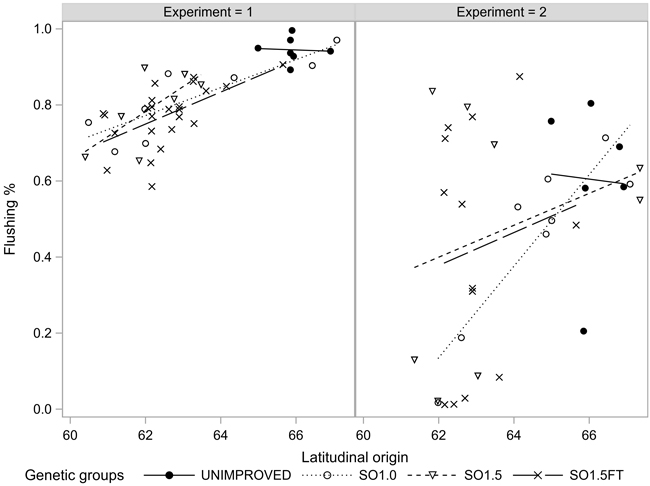
Fig. 6. Bud flushing rate (BUDFLUSH) of the seedlots in the two experiments. The flushing rates were estimated for 100% and 10% injured seedlings in EXP1 and EXP2, respectively.
4 Discussion
We observed a similar relationship between the timing of the growth cessation events (GRCESS and BUDSETDATE) and the geographical origin as demonstrated earlier by Mikola (1982), Hurme et al. (1997), Savolainen et al. (2011) and Andersson Gull et al. (2018). The clinal trend was notably weaker in the growth chamber experiment (EXP1) which also showed a higher frequency of seedlings with secondary needles and other growth anomalies than EXP2. The difference appeared to be due to the northern origins which terminated their growth more than 10 days before the southern origins in EXP2, but almost the same time in EXP1. A plausible explanation for the smaller phenological differences between the southern and northern origins in EXP1 could be the occurrence of some unknown disturbances in the controlled growth conditions. However, we are not aware of malfunctions in growth chamber facilities during the execution of EXP1 and thus cannot give a definite explanation for the different results from the two experiments.
Mikola (1982) reported “some hints” that Scots pine plus-tree progenies of northern origin had a slightly later average bud set compared to the respective natural origins. Our results were somewhat inconsistent. Whereas the southern seed orchard origins in EXP1 appeared to have a slightly earlier phenology than the unimproved materials, none of the among-group differences in either GRCESS or BUDSETDATE were large enough to show as statistically significant. Summing up, our results did not give support to Sarvas’s (1970) speculation that “…many or, maybe, most, of the best plus trees have a long active period…”, i.e., a more “southern” phenology by comparison to unselected materials of the same origin.
Artificial freeze testing of young seedlings (Nilsson 1988; Aho 1994; Hurme et al. 1997; Zhang et al 2003; Savolainen et al. 2004; Dormling and Johnsen 2011; Lehtinen and Pulkkinen 2018) or needles (Nilsson and Walfridsson 1995; Abrahamsson et al. 2012) has become a standard method for the evaluation of autumn frost hardening in Scots pine. Several studies have confirmed the validity of freezing damages as a proxy for long-term genetic differences in hardiness by reporting moderate, positive genetic correlations between freezing injuries in one-year-seedlings and survival and vitality of the same materials in field trials at 10–20 years of age (Nilsson and Eriksson 1986; Nilsson and Andersson 1987; Persson et al. 2010).
Hurme et al. (1997) found the timing of bud set and the development of autumn frost hardiness to be highly correlated at the population level. In our study, the weakish latitudinal trend observed in the growth rhythm traits in EXP1 did not repeat for FRINJURY. Instead, both experiments showed a similar strong latitudinal pattern of variation in autumn frost tolerance like many studies have done earlier (Andersson 1986; Nilsson and Eriksson 1986; Aho 1994; Hurme et al. 1997). Our result suggests that the process of physiological hardening in Scots pine seedlings is partly independent of the timing of visible phenological events.
The differences among the unimproved and improved groups were mostly insignificant except for the relatively poor hardiness of the southern SO1.0 materials in EXP1 and the northern SO1.0 materials in EXP2. This is in line with Andersson (1986) who reported elevated rates of needle injury in Swedish first-generation seed orchard progenies, corresponding to approximately half a degree shift in the latitude of origin. However, we cannot fully exclude the possibility that the below-par hardiness of the SO1.0 materials was an artifact caused by incorrectly determining the latitudinal origins of the seedlots. This origin for each seed orchard seedlot was calculated using a function that assumed pollen contamination to originate from stands surrounding the seed orchard and stabilize at the 40% level in a fully developed seed orchard (Berlin et al. 2019). If the old first-generation orchards were subjected to higher levels of contamination and more southern pollen sources than assumed in the calculations, the seedlots would represent more southern origins than was expected. Bhumibhamon (1978) showed that when plus trees of northern origin are deployed in seed orchards in the southern part of Finland, they start their flowering before the local stands which is likely to subject them to pollen contamination from even more southern stands. On the other hand, the SO1.5 and SO1.5FT seedlots had their origin determined precisely in the same way, yet both appeared consistently hardier than the SO1.0 materials. This speaks against the possibility that the misprediction of seedlot origins would be the main reason for the slightly worse frost hardiness of the SO1.0 materials.
The consistent ranking of the unimproved, first generation and 1.5-generation orchard materials in FRINJURY (Fig. 5) hints of another possibility: that the phenotypically selected plus trees deployed as parents in the first-generation seed orchards have been marginally less hardy than the average wild trees. The following re-selection to the 1.5-generation seed orchards has then eliminated this inferiority. The selection of 1.5-generation plus trees has also involved some hardiness-related traits. Although these traits have received much less emphasis than the growth rate and stem and branch quality (Antola and Hahl 1997), even a mild selection for hardiness may have been sufficient to keep the most susceptible plus trees out of the 1.5-generation seed orchards. However, the suggested course of events should be taken as a working hypothesis rather than a firm conclusion. More data are required to prove the change in hardiness between the two classes of seed orchards in statistically valid terms.
The seed orchards composed of freezing-tested clones of northern origin (SO1.5FT) came out the hardiest genetic group in both experiments, yet this superiority was not statistically significant. The results for the SO1.5FT materials showed an abundant variation among seed crops from different years, reflecting the annual variation in realized pollen contamination levels. The hardiest seed orchard seedlot of this study was, predictably, from the northernmost Scots pine seed orchard (SO1.5FT) in Finland which is located above the 66th degree of latitude where the contaminating pollen is likely to be locally adapted and thus with a negligible effect on adaptive traits.
We assessed the resilience of the materials to freezing damage by recording bud flushing in the damaged seedlings in June following the freezing test. The results for BUDFLUSH implied a weak and statistically non-significant latitudinal cline and non-significant differences among the groups. The materials tested in EXP1 and EXP2 showed, on average, highly divergent flushing rates although the levels of needle injuries were similar. The materials in EXP2 were kept outdoors during the winter following the freezing test. This likely increased the mortality of the stressed seedlings and resulted in the poor overall rate of BUDFLUSH. In EXP1, where the flushing rates were markedly higher than in EXP2, the seedlings did not undergo a dormant period between the freezing test and the BUDFLUSH observations.
Overall, we did not find evidence of alarming compromises in the adaptive performance of genetically improved materials. Despite the controlled conditions in which the experiments were conducted, much of the variation in the studied traits remained unexplained by the statistical model. Considered retrospectively, larger sample sizes would have been necessary to detect the possible differences among the classes of reproductive materials studied here. On the other hand, the results imply that such differences are likely to be subtle and not of practical significance regarding the operational deployment of genetically improved materials.
The 1.5-generation seed orchard materials showed adaptive performance that was well representative of the local populations of the same latitudinal origin. This result essentially complies with the study of MacLachlan et al. (2017), who did not detect any indirect effects from selective breeding on adaptive traits in lodgepole pine (Pinus contorta Douglas ex Loudon). Similarly, Hawkins and Stoehr (2009) did not find a significant trade-off between cold hardiness and growth rate in coastal Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco var. menziesii). Hannerz et al. (1999) predicted fast-growing materials of western hemlock (Tsuga heterophylla (Raf.) Sarg.) to display more damages from spring frosts than unimproved materials whereas for fall frost hardiness they observed no difference between the materials.
The slightly inferior frost hardiness of the first-generation materials was a single aberrant phenomenon to which various explanations are possible. The 1.5-generation orchard materials appeared to be marginally more capable of enduring autumn frosts than the materials from first-generation seed orchards, but we lacked a statistically sound confirmation, and the finding remains to be validated in future studies. We expect that a series of realized gain trials established in 2016 and 2017, which involves many of the seedlots analyzed in this study, may soon give further insight into the results.
Acknowledgements
We want to thank the anonymous reviewers for their constructive comments and suggestions. We are also grateful to the personnel of the Haapastensyrjä Unit of Luke for their participation to the conduct of the two experiments. The Natural Resources Institute Finland (Luke) funded this work through the OPRES (41007-00127000) project.
Authors’ contributions
Both authors participated in the conception of the research questions, planning of the experiments and the interpretation of the results. MH was responsible for data processing and statistical data analyses, and mainly responsible for writing the manuscript. SR selected the research materials and contributed to the contents of the manuscript.
Declaration of openness of research materials, data, and code
Access to the data and other digital materials used in this study is provided by the corresponding author on request.
References
Abrahamsson S, Nilsson J, Wu H, Garcia Gil M, Andersson B (2012) Inheritance of height growth and autumn cold hardiness based on two generations of full-sib and half-sib families of Pinus sylvestris. Scand J For Res 27: 405–413. https://doi.org/10.1080/02827581.2012.663403.
Aho M (1994) Autumn frost hardening of one-year-old Pinus sylvestris seedlings: effects of origin and parent trees. Scand J Forest Res 9: 17–24. https://doi.org/10.1080/02827589409382808.
Andersson B (1986) Freezing tests of Scots pine (Pinus sylvestris L.) seed orchard crops. In: Lindgren D (ed) Provenances and forest tree breeding for high altitudes. Swed Univ Agric Sci Dept For Genet Plant Physiol Rep 6: 99–111.
Andersson B, Elfving B, Persson T, Ericsson T, Kroon J (2007) Characteristics and development of improved Pinus sylvestris in northern Sweden. Can J For Res 37: 84–92. https://doi.org/10.1139/x06-224.
Andersson Gull B, Persson T, Fedorkov A, Mullin T (2018) Longitudinal differences in Scots pine shoot elongation. Silva Fenn 52, article id 10040. https://doi.org/10.14214/sf.10040.
Antola J, Hahl J (1997) Kloonivalinta männyn 1,5-polven siemenviljelykseen (sv 408) Viialan Hinkkaan. [Clone selection for the 1.5-generation seed orchard no. 408 in Hinkka, Viiala]. Metsänjalostussäätiön työraportteja 44: 1–15.
Berlin M, Persson T, Jansson G, Haapanen M, Ruotsalainen S, Bärring L, Andersson B (2016) Scots pine transfer effect models for growth and survival in Sweden and Finland. Silva Fenn 50, article id 1562. https://doi.org/10.14214/sf.1562.
Berlin M, Almqvist C, Haapanen M, Högberg K, Jansson G, Persson T, Ruotsalainen S (2019) Common Scots pine deployment recommendations for Sweden and Finland. Skogsforsk Arbetsrapport 1017: 1–65.
Bhumibhamon S (1978) Studies on Scots pine seed orchards in Finland with special emphasis on the genetic composition of the seed. Commun Inst For Fenn 94: 1–118. http://urn.fi/URN:NBN:fi-metla-201207171125.
Dormling I, Johnsen Ø (1992) Effects of the parental environment on full-sib families of Pinus sylvestris. Can J For Res 22: 88–100. https://doi.org/10.1139/x92-013.
Haapanen M, Hynynen J, Ruotsalainen S, Siipilehto J, Kilpeläinen M (2016). Realized and projected gains in growth, quality and simulated yield of genetically improved Scots pine in southern Finland. Eur J Forest Res 135: 997–1009. https://doi.org/10.1007/s10342-016-0989-0.
Hannerz M, Aitken S, King J, Budge S (1999) Effects of genetic selection for growth on hardiness in western hemlock. Can J For Res 29: 509–516. https://doi.org/10.1139/x99-019.
Hawkins B, Stoehr M (2009) Growth, phenology, and cold hardiness of 32 Douglas-fir full-sib families. Can J For Res 39: 1821–1834. https://doi.org/10.1139/X09-092.
Hurme P, Repo T, Savolainen O, Pääkkönen T (1997). Climatic adaptation of bud set and frost hardiness in Scots pine (Pinus sylvestris L.). Can J For Res 27: 716–723. https://doi.org/10.1139/x97-052.
Jansson G, Hansen J, Haapanen M, Kvaalen H, Steffenrem A (2017) The genetic and economic gains from forest tree breeding programmes in Scandinavia and Finland. Scand J Forest Res 32: 273–286. https//doi.org/10.1080/02827581.2016.1242770.
Lehtinen M, Pulkkinen P (2017) Effects of Scots pine paternal genotypes of two contiguous seed orchards on the budset and frost hardening of first-year progeny. Silva Fenn 51, article id 7783. https://doi.org/10.14214/sf.7783.
Littell R, Milliken G, Stroup W, Wolfinger R, Schabenberger O (2006) SAS for Mixed Models. 2nd edition. SAS Institute Inc, Cary, NC.
MacLachlan I, Wang T, Hamann A, Smets P, Aitken S (2017) Selective breeding of lodgepole pine increases growth and maintains climatic adaptation. Forest Ecol Manag 391: 404–416. https://doi.org/10.1016/j.foreco.2017.02.008.
Mikola J (1982) Bud-set phenology as an indicator of climatic adaptation of Scots pine in Finland. Silva Fenn 16: 178–184. https://doi.org/10.14214/sf.a15075.
Mikola J (1993) Provenance and individual variation in climatic hardiness of Scots pine in Northern Finland. In: Alden J, Mastrantonio J, Ødum S (eds) Forest development in cold climates. Proceedings of a NATO Advanced Research workshop held June 18–23, 1991 in Laugarvatn, Iceland. Plenum Press, New York, pp 333–342.
Natural Resources Institute Finland (2021) https://stat.luke.fi/metsa. Accessed 18 August 2021.
Nilsson J (1988) Variation in the rate of winter hardening of one-year-old plus-tree families of Scots pine raised in different environments. Silva Fenn 2: 213–224. https://doi.org/10.14214/sf.a15511.
Nilsson J, Andersson B (1987) Performance in freezing testing and field experiments of full-sib families of Pinus sylvestris (L.). Can J For Res 17: 1340–1347. https://doi.org/10.1139/x87-207.
Nilsson J, Eriksson G (1986) Freeze testing and field mortality of Pinus sylvestris (L.) in northern Sweden. Scand J For Res 1: 205–218. https://doi.org/10.1080/02827588609382412.
Nilsson J, Walfridsson E (1995) Phenological variation among plus-tree clones of Pinus sylvestris (L.) in Northern Sweden. Silvae Genet: 44: 20–28.
Oskarsson O (1972) Finnish plus trees of Scots pine and Norway spruce. Folia For 150: 1–138. http://urn.fi/URN:ISBN:951-40-0028-5.
Persson T, Andersson B, Ericsson T (2010). Relationship between autumn cold hardiness and field performance in northern Pinus sylvestris. Silva Fenn 44: 255–266. https://doi.org/ 10.14214/sf.152.
Rehfeldt G (1992) Early selection in Pinus ponderosa: compromises between growth potential and growth rhythm in developing breeding strategies. Forest Sci 38: 661–677.
Rosvall O, Jansson G, Andersson B, Ericsson T, Karlsson B, Sonesson J, Stener L (2001) Genetiska vinster i nuvarande och framtida fröplantager och klonblandningar. [Summary: Genetic gains from present and future seed orchards and clone mixes]. Skogforsk, Redogörelse 1: 1–41.
Sarvas R (1970) Establishment and registration of seed orchards. Folia For 89: 1–24. http://urn.fi/URN:NBN:fi-metla-201207191746.
SAS Institute Inc (2013) SAS/STAT® 13.1 User’s guide. Cary, NC.
SAS Institute Inc (2017) SAS/STAT® 14.3 User’s guide. Cary, NC.
Savolainen O, Bokma F, Garcı́a-Gil R, Komulainen P, Repo T (2004) Genetic variation in cessation of growth and frost hardiness and consequences for adaptation of Pinus sylvestris to climatic changes Forest Ecol Manag 197: 79–89. https://doi.org/10.1016/j.foreco.2004.05.006.
Savolainen O, Kujala S, Sokol C, Pyhäjärvi T, Avia K, Knürr T, Kärkkäinen K, Hicks S (2011) Adaptive potential of northernmost tree populations to climate change, with emphasis on Scots pine (Pinus sylvestris L.). J Heder 102: 526–536. https://doi.org/10.1093/jhered/esr056.
Zhang G, Ryyppö A, Vapaavuori E, Repo T (2003) Quantification of additive response and stationarity of frost hardiness by photoperiod and temperature in Scots pine. Can J For Res 33: 1772–1784. https://doi.org/10.1139/X03-100.
Total of 35 references.

