Habitat associations of red-listed epiphytic lichens in Finland
Nirhamo A., Pykälä J., Jääskeläinen K., Kouki J. (2023). Habitat associations of red-listed epiphytic lichens in Finland. Silva Fennica vol. 57 no. 1 article id 22019. https://doi.org/10.14214/sf.22019
Highlights
- We analyzed the habitat associations of 231 nationally red-listed epiphytic lichen species in Finland
- Their habitat associations were varying, but deciduous trees, old forests and trees, and microclimates with intermediate or high light availability and humidity were particularly important
- The maintenance of the habitats of many red-listed epiphytic lichens is difficult if not impossible to combine with intensive forest management.
Abstract
The Finnish red list shows that the epiphytic lichen flora of Finnish forests is highly threatened and declining steeply. Red lists provide limited information on the habitat associations of threatened species, which could be relevant in informing management and conservation measures. We used documented empirical data and expert assessments to determine for each red-listed (IUCN categories Near Threatened, NT; Vulnerable, VU; Endangered, EN; Critically Endangered, CR; Regionally Extinct, RE) epiphytic lichen species of Finland the following key habitat associations: host tree species, substrate type, habitat type, geographical distribution, preferred microclimate, and minimum required forest and tree age. The most important host tree species were Picea abies (L.) H. Karst. and Populus tremula L. Other tree species of high importance included Sorbus aucuparia L. and Salix caprea L. One fourth of red-listed epiphytic lichens were primarily lignicolous. Most species required old-growth forests (required by 41% of species) or old trees (52%), but many species required only mature forests (36%) or trees (35%). The microclimatic preferences of most red-listed epiphytic lichens consisted of high or intermediate light availability and humidity. Most species whose status had deteriorated were dependent on deciduous trees. The continuous availability of old deciduous trees (especially Populus, Salix and Sorbus) requires special attention in both managed and protected forests. Red-listed epiphytic lichens would be aided by increased forest protection or transitioning to less intensive management regimes.
Keywords
boreal forests;
deciduous trees;
biodiversity;
conservation;
threatened species
-
Nirhamo,
School of Forest Sciences, University of Eastern Finland, Joensuu, Finland
 https://orcid.org/0000-0002-1487-533X
E-mail
aleksi.nirhamo@uef.fi
https://orcid.org/0000-0002-1487-533X
E-mail
aleksi.nirhamo@uef.fi

-
Pykälä,
Finnish Environment Institute, Helsinki, Finland
 https://orcid.org/0000-0002-7566-9310
E-mail
juha.pykala@syke.fi
https://orcid.org/0000-0002-7566-9310
E-mail
juha.pykala@syke.fi
- Jääskeläinen, Kuopio Museum of Natural History, Kuopio, Finland E-mail jaaskimmo@gmail.com
-
Kouki,
School of Forest Sciences, University of Eastern Finland, Joensuu, Finland
 https://orcid.org/0000-0003-2624-8592
E-mail
jari.kouki@uef.fi
https://orcid.org/0000-0003-2624-8592
E-mail
jari.kouki@uef.fi
Received 11 November 2022 Accepted 7 March 2023 Published 29 March 2023
Views 85318
Available at https://doi.org/10.14214/sf.22019 | Download PDF

Supplementary Files
1 Introduction
Epiphytic lichens, i.e. lichens that grow commensally on the surface of trees or other plants, are a highly diverse and ecologically distinctive component of the biotic communities of forest ecosystems across the globe (Ellis 2012). Epiphytic lichens are often vulnerable to environmental change, and their decline has been identified in many regions (Nascimbene et al. 2013; Allen et al. 2019; Tagirdzhanova et al. 2019). The main driver of this decline is habitat loss, particularly of old-growth forests (Pykälä et al. 2019; Allen et al. 2019). Lichen diversity has been or is expected to be negatively affected also by other drivers such as pollution and climate change (Hawksworth and Rose 1970; Rubio-Salcedo et al. 2017). The main cause of the decline of epiphytic lichen diversity in Finland is the shift from natural forests to intensively managed forests. This shift has occurred over several centuries but accelerated in the 1950’s when forest use intensified. The silvicultural system that has been in use in Finland since then mostly corresponds to “intensive even-aged forestry”, sometimes with elements of “combined objective forestry”, as described by Duncker et al. (2012). The coverage of old (>150 years) forests in Finland is low at 5.9% of forest area. The distribution of old forests is heavily skewed towards northern Finland, as only 1.7% of forest area in southern Finland consists of old forests (Mönkkönen et al. 2022). Forest management has resulted in profound structural changes in Finnish forests, including the loss of old-growth structures and significant reductions in deadwood volume and in the proportion of deciduous trees. Consequently, there have been drastic changes in the biotic communities of forests (Esseen et al. 1997; Kuuluvainen 2009; Hyvärinen et al. 2019; Mönkkönen et al. 2022).
Red lists provide systematic global or national overviews of the conservation status of species and the threats that they face. National red lists apply the IUCN red list categories and criteria (Mace et al. 2008) to assess the extinction risks of species at the national level. The Finnish red list was most recently compiled in 2019 (Hyvärinen et al. 2019). The status of 1944 lichen taxa from the 2003 lichen taxa known from Finland at the time was assessed, although roughly a fifth of lichen species were assessed as Data Deficient (DD). Concerning species with forests as their primary habitat, lichens are the organism group with the highest proportion of nationally threatened (categories Vulnerable, VU; Endangered, EN; Critically Endangered, CR) species at 23.7%. Altogether, 58.3% of lichen species with forests as their primary habitat are red-listed in Finland. Furthermore, the status of lichens is rapidly deteriorating: 51 forest lichen species had a genuine negative threat category change in the 2019 assessment (Hyvärinen et al. 2019). A genuine negative category change means that the status of a species has deteriorated since the previous assessment, and the category change is not due to changes in data accuracy, methodology or interpretation (IUCN 2022). The Finnish red list reveals scarce information on species-specific habitat associations that may be shared among threatened species. Such information is valuable, as it can improve the understanding of the causes of threat and the possible conservation or management actions that are needed to reduce the extinction risk of threatened species.
The habitat associations of red-listed forest species of Finland have previously been examined by Tikkanen et al. (2006), based on the red list assessment published in 2000. They highlighted that red-listed forest species are associated with highly varied habitats, but half of them were associated with old-growth forests, 60% with deadwood and 60% with coniferous trees. However, in the case of lichens, more species were associated with deciduous trees than with coniferous trees. Furthermore, a much lower proportion of lichen species were associated with deadwood (Tikkanen et al. 2006). Deciduous trees have been noted to be preferential host species also for most red-listed epiphytic lichens in Estonia (Marmor et al. 2017). The red list publication itself also highlights the importance of old deciduous trees (Pykälä et al. 2019). After the study by Tikkanen et al. (2006), the data available for red list assessments and the coverage of assessed species have improved considerably. Furthermore, Tikkanen et al. (2006) spared little attention to lichens as they mainly focused on saproxylic beetles and fungi. The high proportion of threatened species among epiphytic lichens and the strongly negative trend in their threat status development demands special attention to be directed towards epiphytic lichens.
This study aims to provide an overview of the current knowledge of the habitat associations of red-listed epiphytic lichens in Finland. We seek patterns behind the endangerment of epiphytic lichens by using expert assessments and data associated with species records to determine key habitat associations of the epiphytic lichen species that are red-listed in Finland in the 2019 assessment. We analyze the associations of red-listed epiphytic lichens with habitat type, forest age, tree age, microclimate and tree species. Additionally, we examine the proportions of corticolous versus lignicolous red-listed species and of species with different types of geographical distributions within Finland. We pay special attention to species that have undergone a genuine negative category change. Our goal is to draw attention to threatened epiphytic lichens and to reveal both details as well as general patterns in their ecology, which may be used as a base for ecologically informed and efficient management and conservation measures.
2 Materials and methods
2.1 Data acquisition
In this study, we used three types of data. First, we used some basic information such as the names of the species on the Finnish red list, their IUCN categories and category changes that is openly available at The Web Service of the Red List of Finnish Species (https://punainenkirja.laji.fi/en). Second, we used habitat information included in species records. We utilized the species records in the database that was used for the national red list assessments of lichens. This database is not public, but most of these records, although often with reduced information e.g. on host species, are accessible at the Finnish Biodiversity Info Facility (https://laji.fi/en). Third, we used expert assessments (by authors JP and KJ) to define some of the habitat associations that could not be defined properly using species records alone. The expert assessments were based on several decades of field work, and they utilized the highest level of knowledge on these species that is available in Finland. To reduce the risks of uncertainty and misclassifications, the assessments were done on a coarse level (Table 1). Lichen nomenclature followed the red list publication (Pykälä et al. 2019). We also provided up-to-date species names following the checklist of Finnish lichens from the year 2021 (Pykälä et al. 2022).
| Table 1. The attributes we used to describe the habitat associations of red-listed epiphytic lichens in Finland, the sources of information for the attributes, their categorization and the method of assigning the categories to lichen species. | |||
| Source of information | Attribute | Categories | Method |
| Species records | Primary host tree species | Finnish tree species | Tree species with the most records of the lichen species |
| Secondary host tree species | Finnish tree species | Up to three tree species with a considerable amount of records of the lichen species | |
| Substrate | Corticolous, Lignicolous, Lichenicolous | The substrate with the most records of the lichen species | |
| Distribution | Hemiboreal, Southern, Northern, Wide latitudinal range | See text | |
| Expert assessments | Habitat type | The classification in the Finnish red list (Hyvärinen et al. 2019) | Preferred conditions |
| Forest age | Young (<60 yrs), Mature (60–120 yrs), Old (>120 yrs), Indifferent | Minimum age class required by the lichen species | |
| Tree age | Young (<60 yrs), Mature (60–120 yrs), Old (>120 yrs) | Minimum age class required by the lichen species | |
| Humidity | Dry, Intermediate, Humid | Preferred conditions | |
| Light availability | Low, Intermediate, High | Preferred conditions | |
In the Finnish red list, lichens are grouped together with lichen-allied fungi which include lichenicolous fungi and morphologically lichen-like saprotrophic fungi that have traditionally been studied by lichenologists. Out of the 2003 assessed species of lichens and lichen-allied fungi, 291 were lichen-allied fungi. Lichen-allied fungi were included in this study, but in this text, lichens and lichen-allied fungi are collectively referred to as lichens for the sake of brevity.
We compiled a list of all red-listed (IUCN categories Near Threatened, NT; Vulnerable, VU; Endangered, EN; Critically Endangered, CR; Regionally Extinct, RE) Finnish species of lichens that utilize trees or wood as their primary substrate, i.e. species that are epiphytic. Species in the category DD (Data Deficient) were excluded, because most of these species were too poorly known to make an adequate assessment of their habitat associations. For the same reason, we excluded all species in any category with 5 or less records in the database from the analyses. We also delineated a subset that included the species that had undergone a negative genuine category change either in the assessment of 2010 (Rassi et al. 2010) or 2019 (Hyvärinen et al. 2019). Both assessments were included in order to obtain a larger pool of species that have been observed to decline in the recent past.
2.2 Assignment and classification of habitat associations
We defined primary and secondary habitat types, primary and secondary host tree species, substrate type, the minimum age class of host forest and host tree, preferential microclimate and geographical distribution for each nationally red-listed epiphytic lichen species with a sufficient number of records in Finland. We examined species records to define primary and secondary host tree species, primary substrate and geographical distribution of the red-listed epiphytic lichens. We used expert assessments to define preferred habitat types and microclimatic conditions (humidity and light availability), and the minimum required forest age and tree age (Table 1). The tree species with the most records of a given lichen species was assigned as the primary host. Similarly, up to three tree species that were next in the ranking in the number of records of the lichen species were assigned as secondary hosts, if the records were clearly not only sporadic. If the primary substrate of a lichen species was not trees (wooden structures or non-tree plants), no primary host tree species was assigned. If a primary host for a species could not be distinguished because records were evenly distributed between several tree species, no primary host was assigned, and up to three most common hosts were assigned as secondary hosts. The thresholds of the age classes were set on the basis that “old” (>120 years) ages are not covered by standard forest management in Finland, “mature” (60–120 years) ages are covered to a limited but varying extent by forest management as rotation lengths vary by latitude and site fertility, and “young” (<60 years) ages are fully covered by forest management. The forest habitat type classification of the Finnish red list divides forests into xeric, mesic and herb-rich forests, following the classification by Cajander (1949). In the case of lichens, this forest categorization was utilized sparingly, and in this study we narrowed the preferential forest habitat types of red-listed epiphytic lichens down to xeric, mesic and herb-rich forests based on expert assessments. Aside from the increased detail in the classification of forest types, the habitat types of species are derived directly from the Finnish red list (https://punainenkirja.laji.fi/en).
Due to a lack of data, classifications regarding microclimate (humidity and light availability) are not based on objective measurements, but on inference from stand structure, topography and hydrological conditions. Levels of humidity and light availability were split into three categories which aimed to cover a similar portion of the gradients in these variables. Examples of habitats with specific types of microclimates include dense spruce stands for low light availability and high humidity; dense pine stands, sparse spruce stands and many edge habitats for intermediate conditions; sparse pine stands and clearcuts or other sites of major disturbance and for high light availability and low humidity. The inferred levels of humidity and light availability are often correlated, but we also recognized some situations where we considered this not to be the case. These include old-growth spruce stands with canopy gaps, where light availability is higher but humidity may be maintained, and stands near bodies of water where both humidity and light availability may be high.
The distribution of a species was considered as northern, if it had been observed only in the biogeographical region of Kainuu and/or to the north of it; southern, if it had been observed only in Kainuu and/or to the south of it; hemiboreal, if it had been observed only in the biogeographical regions of Ahvenanmaa, Varsinais-Suomi and/or Uusimaa. Species that occurred both to the south and north of Kainuu were considered to have a wide latitudinal range. In the few cases where a species had been observed only in Kainuu, the species was considered to have a wide latitudinal range.
In all the classifications, we used the category “unknown” if there was an insufficient amount of data available or if we were unable to reach an adequate level of reliability with the expert assessments. When there were only a few records of a species from Finland, in some cases we were able to rely on information from neighboring countries to make adequately reliable assessments of the habitat associations of such species. This was done only when the information from neighboring countries was congruent with the limited data from Finland.
3 Results
The most recent published red list of Finland (Hyvärinen et al. 2019) included 308 epiphytic lichen species in the categories NT, VU, EN, CR and RE. Out of these, 231 species had been observed frequently enough to be included in the analyses. In addition, there were approximately 107 species of epiphytic lichens classified as Data Deficient (DD) (Supplementary file S1). Out of these 231 species, 28 were lichen-allied fungi and 203 were lichens. The number of analyzed species per IUCN category were 100, 81, 31, 15 and 4 for NT, VU, EN, CR and RE, respectively. The number of species excluded from the analyses due to infrequent records per IUCN category were 7, 16, 15, 14 and 25 for NT, VU, EN, CR and RE, respectively.
Several tree species were primary or secondary hosts to red-listed lichens but there were also major differences in the number of lichens hosted (Fig. 1). Overall, 66.7% of red-listed epiphytic lichen species for which we assigned a primary host were primarily hosted by deciduous trees. However, the most important host to red-listed lichens was the coniferous Picea abies (L.) H. Karst. Populus tremula L. was the second most important host. The number of species hosted by a tree species was related to the total volume of the tree species in Finland (Fig. 2). The residuals of each tree species in this correlative comparison may be interpreted as an indicator of how the importance of the tree species differs from what may be expected based on its total volume in Finland, so that positive residuals suggest that the species has higher importance than expected from its total volume, and negative residuals suggest the opposite. Picea abies and Populus tremula have the highest positive residuals. Other tree species with a combination of a positive residual and a high number of hosted species are Sorbus aucuparia L. and, to a lesser extent, Salix caprea L. (Table 2).
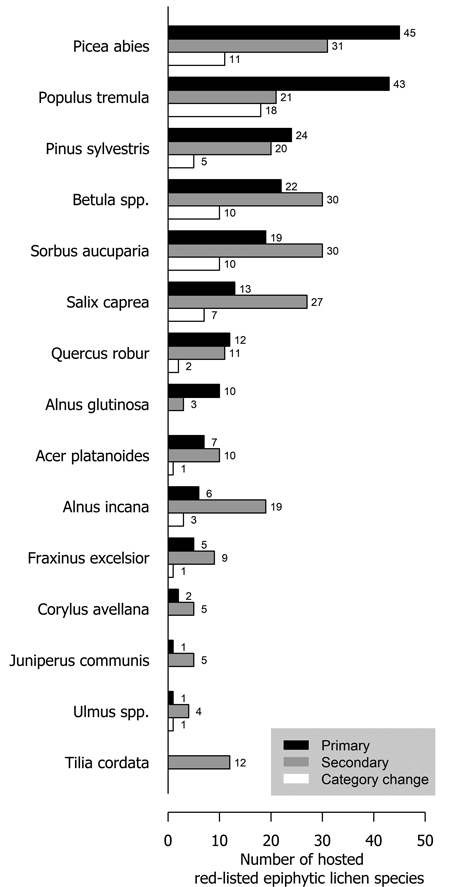
Fig. 1. The number of primarily (black bars) and secondarily (grey bars) hosted red-listed epiphytic lichen species hosted by different tree species in Finland. The species that have undergone a genuine negative threat category change are represented by the white bars. The white bars (species that have undergone a negative category change) are a subset of the black bars (primarily hosted species). Primary host could not be assigned to 21 species (see Methods section).
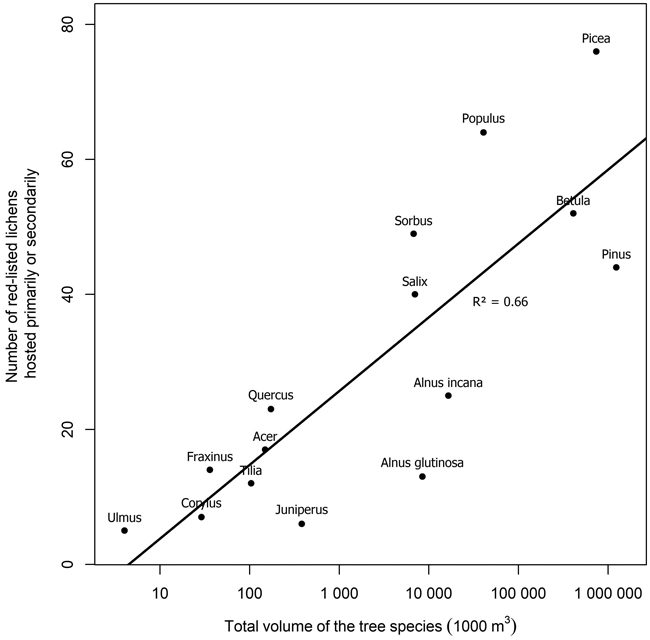
Fig. 2. The relation of the number of red-listed epiphytic lichens hosted primarily or secondarily by a tree species to the total volume of the tree species in Finland (total volumes of tree species from NFI data, Korhonen et al. 2021a). Note that the x-axis is logarithmic. The regression line represents the expected number of primarily hosted species based on the total volume of a tree species.
| Table 2. The residuals (i.e. the distance from the regression line) of each tree species in Fig. 2. The residuals originate from a linear regression where the number of red-listed epiphytic lichens hosted primarily or secondarily by a tree species was the dependent variable, and the total volume of the tree species in Finland was the independent variable. The regression line (i.e. residual value 0) represents the expected number of primarily hosted species based on the total volume of a tree species, and positive residuals indicate that the tree species hosts more red-listed epiphytic lichen species than expected from its total volume in Finland, while negative residuals indicate the opposite. | |
| Tree species | Residual |
| Populus | 20.70 |
| Picea | 18.95 |
| Sorbus | 14.23 |
| Quercus | 5.64 |
| Ulmus | 5.52 |
| Salix | 5.07 |
| Fraxinus | 4.09 |
| Acer | 0.35 |
| Corylus | –1.88 |
| Betula | –2.26 |
| Tilia | –2.94 |
| Alnus incana | –14.01 |
| Juniperus | –15.11 |
| Pinus | –15.51 |
| Alnus glutinosa | –22.84 |
The majority of red-listed epiphytic lichens were corticolous, i.e. growing on the bark of mainly living trees. Lignicolous species, i.e. growing on the wood of mainly dead trees, accounted for a quarter of red-listed epiphytic lichens. The remaining 10 species were lichenicolous (Table 3a). These species were fungi that are parasitic on lichens, and they are a part of the group of lichen-allied fungi. Standing deadwood was preferred by a higher number of species than downed deadwood. In addition, wooden structures were the preferred substrate for some lignicolous species (Table 3b).
| Table 3. The number of red-listed epiphytic lichens and epiphytic lichen species that have undergone a genuine negative threat category change in Finland by a) primary substrate type, b) primary deadwood type (lignicolous species), c) geographical distribution. One species had resin as its substrate, and it is not included in a). | ||||
| Number of species | % | No. of category change species | % | |
| a) Substrate | ||||
| Corticolous | 163 | 70.6 | 56 | 77.8 |
| Lignicolous | 57 | 24.7 | 15 | 20.8 |
| Lichenicolous | 10 | 4.3 | 1 | 1.4 |
| b) Deadwood type | ||||
| Downed | 19 | 33.3 | 3 | 20.0 |
| Standing | 28 | 49.1 | 10 | 66.7 |
| Wooden structures | 9 | 15.8 | 2 | 13.3 |
| Unknown | 1 | 1.8 | 0 | 0.0 |
| c) Distribution | ||||
| Hemiboreal | 24 | 10.4 | 4 | 5.6 |
| Southern | 71 | 30.7 | 17 | 23.6 |
| Northern | 11 | 4.8 | 4 | 5.6 |
| Wide range | 125 | 54.1 | 47 | 65.3 |
Mesic forests were the preferred habitat type for more than half (57.6% of analyzed species) of red-listed epiphytic lichens. Herb-rich forests also harbored a high number of species (23.4%). Much fewer species (8.2%) preferred xeric forests. In total, 25 species preferred habitat types that are not considered forests in the habitat type classification of the Finnish red list. These were mostly classified as shore habitats (forests on the shores of streams, lakes or the sea), mire habitats (wooded mires) and rural biotopes (wooded agricultural land). Only parks and buildings with a total of 11 species are strictly non-forest habitat types (Fig. 3).
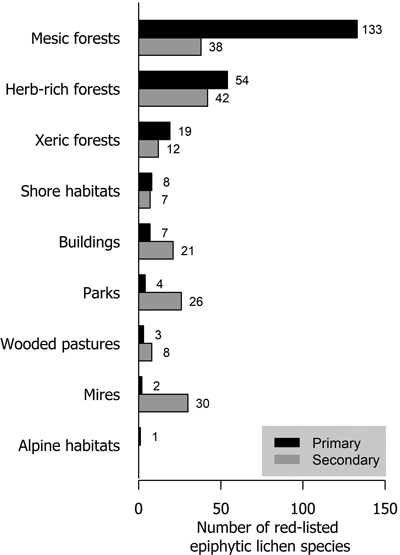
Fig. 3. The number of red-listed epiphytic lichen species by primary (black bars) and secondary (grey bars) habitat types.
Most red-listed epiphytic lichens required either old-growth forests (41.1% of analyzed species) or mature forests (36.3%). Very few species (3.0%) were inhabitants of young forests. Additionally, we considered some species (16.0%) to be indifferent to forest age. Old trees were required by a larger portion of species (51.5%) than old forests. The minimum required tree age class was mature for many species (35.1%) and young for some species (9.5%) (Fig. 4).
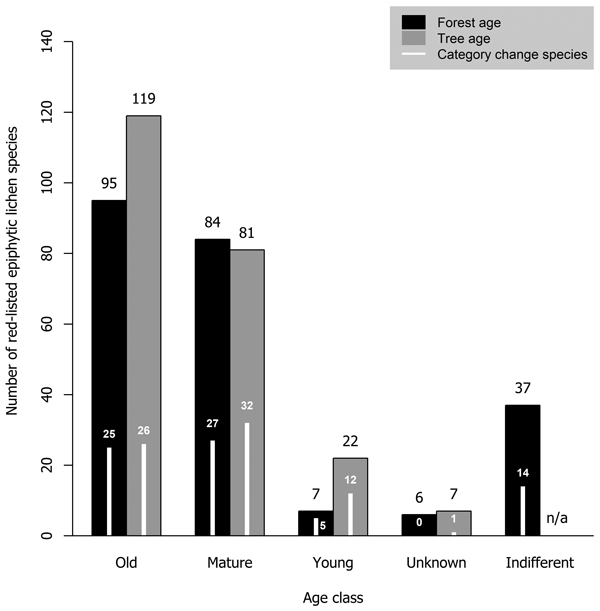
Fig. 4. The number of red-listed epiphytic lichens by minimum age class requirement concerning forest age (black bars) and tree age (grey bars), and the number of species that have undergone a genuine negative threat category change (white bars) by forest and tree age class. Forest age and tree age were not applicable to two species (one of them had undergone a category change) that have been observed only on wooden structures in Finland.
Very few red-listed epiphytic lichens (3.9% of analyzed species) preferred a dry microclimate. Most species preferred intermediate (52.8%) or high humidity (37.6%). Most red-listed epiphytic lichens (57.1%) preferred intermediate light availability, while also high light availability was preferred by many (31.2%), and low light availability by some (8.7%). When preferences for both humidity and light availability were examined together, most species (80.5%) preferred some combination of intermediate or high humidity and light availability (Table 4).
| Table 4. The number of a) red-listed epiphytic lichens, b) species that have undergone a genuine negative threat category change, with various microclimatic preferences concerning light availability (vertical) and humidity (horizontal). | |||||
| a) | Dry | Intermediate | Humid | Unknown | |
| High | 9 | 33 | 24 | 6 | 72 |
| Intermediate | 0 | 82 | 47 | 3 | 132 |
| Low | 0 | 7 | 13 | 0 | 20 |
| Unknown | 0 | 0 | 3 | 4 | 7 |
| 9 | 122 | 87 | 13 | ||
| b) | Dry | Intermediate | Humid | Unknown | |
| High | 2 | 11 | 4 | 0 | 16 |
| Intermediate | 0 | 39 | 10 | 1 | 48 |
| Low | 0 | 2 | 3 | 0 | 5 |
| Unknown | 0 | 0 | 0 | 0 | 0 |
| 2 | 50 | 17 | 1 | ||
Most red-listed epiphytic lichens had a wide latitudinal range. Species with a southern or a hemiboreal distribution were much more numerous than those with a northern distribution (Table 3c).
A total of 73 epiphytic lichen species underwent a negative genuine category change in the assessments of 2010 or 2019, so that 18, 44 and 11 species had a category change in the assessment of 2010, 2019, or both assessments, respectively. One of them was excluded from the analyses due to infrequent records. A higher percentage of species hosted by deciduous tree species with a wide range within Finland had undergone a genuine negative category change than of those hosted by coniferous tree species or the deciduous tree species restricted to the hemiboreal zone (Fig. 1). There were species preferring each age class among the species that had undergone a genuine negative category change, but species preferring mature age classes were the most numerous by a slight margin over the old age classes (Fig. 4). Species that had undergone a category change mostly preferred intermediate humidity and light availability; the percentage of species that preferred high humidity or high light availability was lower here, than among the total sum of red-listed epiphytic lichens (Table 4).
4 Discussion
Parts of our study were based on expert assessments, which is a method that includes uncertainty and subjectivity. To complete our study objectives, expert assessments were the only viable option since extensive, objective data is missing for many of the habitat associations we analyzed for most of the analyzed species. We excluded species with infrequent records from the analyses to limit the level of uncertainty in the assessments of habitat associations. We did, however, complete the habitat association assessments also for these species. Including these species in the analyses would have had very little impact on the conclusions to be drawn from the results. This reaffirms our view that when the sum of all species was analyzed, the uncertainties at the level of individual species were diminished, and that the assessments made in this study were adequately reliable in relation to our study objectives.
4.1 Tree species
Several tree species were primary or secondary hosts to a noteworthy amount of red-listed lichen species, even though there were clear differences between tree species in the number of hosted red-listed lichens. Tree species may differ as lichen hosts due to differences related to bark chemistry (e.g. pH), bark texture and structure, age span, decay dynamics, habitat specialization, successional niches and geographical distribution (Kuusinen 1996; Ellis 2012).
The number of red-listed lichens primarily or secondarily hosted by a tree species was explained to a large extent by the total volume of the tree species in Finland. This is partially a consequence of the procedure we used to assign primary and secondary hosts, which favored common tree species. We assigned the primary host of a lichen species to be the tree species with the highest number of records of the lichen species. A less common tree species could have been a more favorable host to a given lichen species but not be assigned as its primary or even secondary host, because the species had been recorded more often on more common trees. Therefore, these results do not indicate the ability of various tree species to host red-listed lichens, rather, they indicate the realized relative importance of tree species as hosts of red-listed lichens in Finland.
Both the numbers of hosted species and the residuals from the expected number of hosted species based on total volume suggest that the most important host tree species for red-listed epiphytic lichens in Finland are Picea abies and Populus tremula. Some red-listed lichens are supported by the branches of Picea abies (e.g. Alectoria sarmentosa (Ach.) Ach., Evernia divaricata (L.) Ach.), and other Finnish tree species do not have similarly lichen-bearing branches. Additionally, deadwood originating from Picea abies is favorable to many lignicolous species. Populus tremula is commonly acknowledged as a key species in boreal Fennoscandia since it provides habitat for a wide variety of specialized associated species (Kivinen et al. 2020). Populus tremula has a relatively high bark pH, which is the main mechanism that differentiates it as a lichen host from the three dominant species of the region (Picea abies, Pinus sylvestris L., Betula spp.) (Kuusinen 1996).
The number of species hosted by Sorbus aucuparia and, to a lesser extent, Salix caprea, was rather high in absolute terms and high in relation to their total volume in Finland. Salix caprea is often mentioned along with Populus tremula as an important host to lichens (Kuusinen 1996; Esseen et al. 1997). In contrast, the value of Sorbus aucuparia as a host of lichens of conservation concern is highly overlooked (but see Pykälä et al. 2006). Both of these tree species have a relatively high bark pH and they often were secondary hosts to lichen species primarily hosted by Populus tremula (e.g. Lobaria pulmonaria (L.) Hoffm. and other cyanolichens). However, Sorbus aucuparia has a distinguishing feature in its smooth bark, which appears to be preferred or required by several red-listed species. These species were typically shared by Sorbus aucuparia and Alnus incana, another tree species with smooth bark. Additionally, while individual deciduous tree species that mainly occur the hemiboreal zone (Quercus robur L., Acer platanoides L., Fraxinus excelsior L., Tilia cordata Mill., Ulmus spp.) were not among the most important hosts of red-listed lichens, as a group they were highly important, despite their minuscule total volume in Finland.
Pinus sylvestris, the most common tree species in Finland, hosted few red-listed lichens in relation to its total volume. Pinus sylvestris hosted few corticolous lichens (see also Kuusinen 1996), but it was the primary host to a higher number of lignicolous lichens than any other tree species: 24 out of 30 species hosted primarily by Pinus sylvestris were primarily lignicolous. Pinus sylvestris provides hard wooden substrates with immense longevity (Niemelä et al. 2002) which is favorable to many lignicolous lichens (Santaniello et al. 2017). Picea abies also hosted a high number of lignicolous species, whereas only a few lignicolous species were hosted primarily by deciduous trees. The slower decomposition rate and thus higher longevity of coniferous wood compared to deciduous wood (Mäkinen et al. 2006) is most likely the main reason why coniferous wood is a more suitable substrate for lignicolous lichens.
The host specificity of epiphytic lichens is usually low (Schmitt and Slack 1990; Cáceres et al. 2007; Rosabal et al. 2013), although it may be higher in Fennoscandia, where tree species richness is low. About one out of three species exhibited a distinct preference to a certain tree species and were not assigned any secondary host species. Most of them had still been recorded on more than one tree species. As such, our study indicates that strict host specificity is rare among red-listed epiphytic lichens of Finland, although affiliations with certain tree species are abundantly clear (Kuusinen 1996).
4.2 Habitat types
About 40% of red-listed forest species in Finland primarily inhabit herb-rich forests (Hyvärinen et al. 2019). These include species that are dependent on hemiboreal tree species (e.g. saproxylic organisms and herbivorous insects) or fertile soils (vascular plants and mycorrhizal fungi), which are red-listed mainly because of the scarcity of herb-rich forests in Finland. Only 25% of red-listed epiphytic lichens inhabited herb-rich forests. The faster turnover of trees in some types of herb-rich forests may disfavor lichens that generally prefer slower habitat dynamics. Additionally, herb-rich forests may have a higher level of shade due to lusher vegetation. However, herb-rich forests are often abundant in deciduous trees, which may be favorable for epiphytic lichen diversity. Especially hemiboreal tree species (Quercus, Acer, Fraxinus etc.) are associated with herb-rich forests in Finland. Our results show a stark contrast between xeric and mesic forests in the richness of red-listed epiphytic lichens. The main reason for this is the low tree species diversity of xeric forests, as it is the primary habitat type only for Pinus sylvestris. All other tree species primarily inhabit mesic or herb-rich forests.
Parks and the artificial substrates of buildings and other structures were primary habitat types for few but secondary for many red-listed epiphytic lichens. Parks typically have old trees, but their removal for safety reasons (Terho and Hallaksela 2008) limits their availability for lichens. Furthermore, because of nitrogen deposition and the legacy effects of historic sulphur deposition (Llewelyn et al. 2020), red-listed lichens are very scarce in parks. Urban forests have been shown to be valuable habitats e.g. for polypores (Korhonen et al. 2021b), but air pollution most likely diminishes the value of urban forests for lichens.
Some lichen species had been observed mostly or exclusively on wooden structures in Finland (see also Svensson et al. 2005). We presume that their natural substrate would be the hard, long-lasting lignum of Pinus sylvestris. However, such substrates (i.e. kelo trees) have been all but eradicated from most of Finland, especially in the southern parts (Niemelä et al. 2002). Thus, these species have been able to persist only on wooden structures such as old barns, sheds etc., which have also been vanishing in the past decades.
4.3 Forest and tree age
We found that 41% of red-listed epiphytic lichens of Finland require old-growth forests. The number of species that require mature forests was only slightly lower. Importantly, for forest and tree age we determined the minimum required age class of species rather than their preferred conditions. As such, it should be noted that it is possible for species that require mature forests to be present in old-growth forests, but not vice versa. Species with mature forests as their minimum required age class may even prefer old-growth forests over mature forests. The requirements on forest or tree age were related to host tree preferences: the lichens with coniferous trees as primary hosts were classified as requiring old-growth forests or trees much more often than the lichens with deciduous trees as primary hosts. There could be two explanations for this. Firstly, managed forests typically cover, at least partially, the ages that we defined as mature, but not those we defined as old. Thus, managed forests could be able to support lichens that require mature coniferous trees but struggle to support lichens that require mature deciduous trees, since they tend to be scarce in managed forests (Siitonen et al. 2000). Secondly, some deciduous tree species (e.g. Populus tremula) have a limited presence in old-growth forests (Lilja et al. 2006). Thus, many lichens that prefer these tree species are associated with mature forests instead, where their preferred host species are more likely to be present.
Tikkanen et al. (2006) highlighted that apart from old-growth forests, also earlier successional stages (i.e. “mature” stands) that are dominated by deciduous trees may be important for red-listed forest species, which our study agrees with. Unmanaged young and mature forests are typically dominated by deciduous trees (Angelstam and Kuuluvainen 2004). However, only about 5.6% of Finnish forests in ages we classified as mature (60–120 yrs) are dominated by deciduous trees (data from NFI: Korhonen et al. 2021a; see also Mönkkönen et al. 2022), since conifers are favored in forest management for commercial reasons. A high presence of deciduous trees in young or mature forests is likely to increase their conservation value, since they are able to provide habitat for a wider range of red-listed lichens as they develop into high quality habitats over the next decades.
4.4 Microclimate
Epiphytic lichens have widely varied microclimatic preferences (Ellis 2012), but only a few red-listed species preferred conditions with low humidity or light availability, while intermediate or high light availability or humidity were predominantly preferred. Lichens require sufficient humidity (i.e. a hydrated state) and light for photosynthetic activity (Palmqvist 2000). In forests, these features are typically negatively correlated (Chen et al. 1993; Grimmond et al. 2000). Thus, the occurrence of many lichens, as indicated by our results, may be dependent on intermediate conditions where both humidity and light availability are at least moderate (Gauslaa et al. 2006). Our data suggests that some species prefer an unusual combination of high humidity and high light availability (see also Nilsson et al. 2022). Our results highlight that dense forests are inhospitable to many red-listed lichens (see also Gauslaa et al. 2006; Gauslaa et al. 2007; Klein et al. 2020; Nirhamo et al. 2021). Instead, they require a sufficient availability of light, often in combination with relatively high humidity.
Forest stocks in Finland have multiplied by 1.7 over the past century (NFI data: Korhonen et al. 2021a). This is likely to mean that Finnish forests have changed structurally to be denser and to have a higher canopy cover. Long-term changes in canopy cover have been only scarcely studied, but it has been shown to have increased between 1959 and 2011 in unmanaged landscapes in northern Finland (Kulha et al. 2020). Studies have shown that lichen richness is lower in dense stands with low light availability (Marmor et al. 2012; Klein et al. 2020; Nirhamo et al. 2021). According to our results, over 80% of red-listed epiphytic lichens prefer high or intermediate light availability. These species are expected to be negatively affected by high tree density in forests. Thus, increased tree density of Finnish forests is likely to have contributed to the decline of epiphytic lichens.
4.5 Geographical distribution
The species richness of red-listed epiphytic lichens was much higher in southern than in northern Finland. This is in line with typical latitudinal diversity gradients where species richness decreases towards higher latitudes (Hillebrand 2004). Alternatively, southern species could be more likely to be red-listed because of a longer history of forest management, higher air pollution and a higher rate of conversion to agricultural land in southern than in northern Finland. Importantly, survey efforts have been much lower in northern Finland, both historically and recently. Thus, it is very likely that several rare epiphytic lichens remain undiscovered from northern Finland.
4.6 Species with a genuine negative threat category change
A high proportion of lichen species with a genuine negative threat category change were hosted by boreal deciduous trees. Many of these species are affected by the ongoing disappearance of deciduous trees from the remnant old-growth forests of Finland (Hardenbol et al. 2020). This is, on one hand, a result of natural succession patterns (Lilja et al. 2006), but it is also crucially influenced, especially on the landscape-scale, by increased herbivory and disturbance regime alterations which have hindered the recruitment of deciduous trees (Linder et al. 1997; Lankia et al. 2012; Myking et al. 2013; Komonen et al. 2020). The disappearance of deciduous trees from old-growth forests leads to local extinctions of lichens that are hosted by these trees (Snäll et al. 2005; Fedrowitz et al. 2012). On the national level, this has caused significant declines of many lichen species (e.g. Lobaria pulmonaria and other cyanolichens), which are expected to continue in the next decades. Additionally, several species hosted by young deciduous trees have undergone a genuine negative category change. The reasons for this are less clear, but nitrogen deposition is presumed to have affected the recent decline of these species (Pykälä et al. 2019).
Among forest species, lichens appear as a group with an overwhelmingly negative trend concerning genuine category changes (Hyvärinen et al. 2019). We propose four mechanisms which could explain the high frequency of genuine negative category changes in lichens in comparison to other organism groups.
First, lichens seem to be dependent on old deciduous trees more than other organism groups. Many lichens are associated also with Sorbus aucuparia and Salix caprea, which have e.g. very few associated or specialized saproxylic species. Thus, lichens could be more heavily affected by the decline of deciduous trees in old-growth forest reserves.
Second, air pollution is a major cause of threat among red-listed forest lichens, but not among other forest species (Hyvärinen et al. 2019). The effects of air pollution on epiphytic lichens are well documented (although poorly quantified in Finland), and lichen populations have been weakened in the past by sulphur deposition and are negatively affected by nitrogen deposition in the present day (Hawksworth and Rose 1970; Giordani et al. 2014).
Third, many red-listed epiphytic lichens are dependent on high light availability while most other red-listed forest species are not. Thus, lichens may be more sensitive to increased tree density than other organism groups.
Fourth, lichens have exceptionally slow colonization-extinction dynamics (Johansson et al. 2012; Fedrowitz et al. 2012; Johansson et al. 2013a, Johansson et al. 2018). Slow extinction dynamics lead to an accumulation of extinction debt. Thus, extinctions of lichen populations in the present day may be occurring because of environmental changes in the past, after a longer delay than in many other organism groups (Berglund and Jonsson 2005; Ellis and Coppins 2007; Johansson et al. 2013b). Additionally, slow colonization dynamics cause lichens to be slow to respond to management aiming to improve biodiversity. This also contributes to the lack of climate change-related positive category changes among southern species, which have been numerous e.g. in Lepidoptera (Hyvärinen et al. 2019).
4.7 Implications for management
The main cause of threat for epiphytic lichens is the loss of old-growth forests (Pykälä et al. 2019). Thus, the protection of remnant old-growth forests is obviously crucial for halting the decline of red-listed lichens. Currently, protected areas in Finland are heavily skewed towards sites with poor productivity (comparable to or even less productive than the xeric forests of this study) and northern Finland (Korhonen et al. 2021a), in accordance with a global bias in the location of protected areas towards remote sites with poor productivity (Joppa and Pfaff 2009). The overwhelming majority of red-listed epiphytic lichens inhabited mesic or herb-rich forests, and a much higher share had a southern than a northern distribution. This suggests high ineffectiveness of the current network of protected forests in providing habitat to red-listed epiphytic lichens. Efforts should be taken to increase the protection of southern high-productivity forests (Hanski 2000).
Our study showed that many red-listed epiphytic lichens are hosted by deciduous tree species that seem to not persist indefinitely in protected forests (Linder et al. 1997; Lilja et al. 2006; Hardenbol et al. 2020). Simultaneously, these lichens often require forest or tree ages that are higher than those found in managed forests. Thus, the protection of species with these habitat associations is a great challenge. Indeed, many such species have had a recent genuine negative category change, indicating that their protection is failing. So that protected areas may support these species in the long term, they should provide a continuous supply of suitable host trees (Snäll et al. 2005). This would require securing the recruitment of deciduous trees by reducing browsing pressure and reintroducing natural disturbance regimes to protected forests (Lankia et al. 2012; Hardenbol et al. 2020). The recruitment of deciduous trees may also be assisted by interventions in the succession of protected forests (Hämäläinen et al. 2020).
Retention forestry in the managed landscape may compensate for the disappearance of deciduous trees in protected forests, especially with Populus tremula (Gustafsson et al. 2020). However, only limited benefits from the current practices of retention forestry to red-listed epiphytic lichens have been observed in Finland. If lichens of conservation concern are present in pre-harvest forests, their survival on retained trees is high in the short term, but they may face extinctions in the longer term (Perhans et al. 2009; Johansson et al. 2018). However, most of the time red-listed epiphytic lichens are nearly or completely absent from pre-harvest managed forests (Klein et al. 2020; Petersson et al. 2022). Then, colonization of retention trees by lichens of conservation concern is very rare (Lõhmus and Lõhmus 2010). For example, a complete failure by Lobaria pulmonaria to establish onto retained aspens, even when assisted by propagule spraying, has been reported (Belinchón et al. 2017). The very low average retention level in Finland hinders the capability of retention forestry to safeguard biodiversity (Kuuluvainen et al. 2019). The high mortality of retention trees further reduces the effective retention level for epiphytic lichens (Lõhmus and Lõhmus 2010; Rosenvald et al. 2019), which mainly depend on living trees. Furthermore, solitary retention trees or small groups are eventually strongly shaded by the dense regenerating tree cover, which is unfavorable for epiphytic lichens. Since current practices of retention forestry seem to be inadequate in achieving conservation targets, we propose that in addition to increasing strict forest protection, a transition from current practices towards forest management regimes where ecological ambitions are comparable in importance to wood production such as “closer-to-nature” (Larsen et al. 2022) or “natural disturbance-based” (Kuuluvainen et al. 2021) should take place in order to support red-listed epiphytic lichens. This may even be necessary for the success of lichen conservation due to the observed struggle of deciduous trees to regenerate in strictly protected forests.
According to Tikkanen et al. (2006), the majority of red-listed forest species in Finland are dependent on deadwood. Here, only a minority of red-listed epiphytic lichens were primarily lignicolous, and even fewer are strictly dependent on deadwood. Ongoing efforts to increase deadwood volume in support of saproxylic species will most likely support lignicolous lichens as well. However, species that are dependent on long-lasting standing deadwood (i.e. kelo trees; Santaniello et al. 2017) are very likely to require special attention because of the extremely slow formation of their habitat. In addition, epiphytic lichens are more often associated with deciduous trees than other red-listed forest species (Tikkanen et al. 2006). Thus, epiphytic lichens are ecologically distinctive from other groups of red-listed forest species. This implies that species groups require different kinds of habitat management for optimal diversity outcomes (see also Klein et al. 2020). Our results indicate that red-listed epiphytic lichens would be supported by management that maintains a high tree species diversity and high volumes of various deciduous species, provides high quantities of old living trees preferably located in old forests, and avoids high forest density. These guidelines are in conflict with the maximization of wood production, and partially in conflict with the maximization of carbon stocks. As such, the maintenance of the habitats of many red-listed epiphytic lichens is difficult if not impossible to combine with intensive forest management. The status of red-listed epiphytic lichens would benefit from increased forest protection or transitioning to less intensive management regimes. The continuous availability of old deciduous trees requires special attention in both managed and protected forests.
Declaration of openness of research materials, data, and code
The data used for the analyses of this study, acquired by expert assessments and the examination of species records, are available in the Supplementary file S1. Finnish red list data available at The Web Service of the Red List of Finnish Species, https://punainenkirja.laji.fi/en.
Authors’ contributions
AN: Conceptualization, Data acquisition (species records), Data analysis, Interpretation of results, Writing – original draft.
JP: Data acquisition (expert assessments), Writing – revisions.
KJ: Data acquisition (expert assessments), Writing – revisions.
JK: Conceptualization, Writing – revisions, Supervision.
Acknowledgements
We are grateful to Heini Rämä, Annina Kantelinen, Orvo Vitikainen and Arto Puolasmaa who assisted authors JP and KJ in the national IUCN assessment of lichens in Finland. We also express our gratitude to all lichen professionals and enthusiasts in Finland who have made observation records of lichens over the years. We thank Piret Lõhmus and an anonymous reviewer for their helpful comments.
Funding
This study was funded by Maj and Tor Nessling Foundation (personal grant to AN).
References
Allen JL, McMullin RT, Tripp EA, Lendemer JC (2019) Lichen conservation in North America: a review of current practices and research in Canada and the United States. Biodivers Conserv 28: 3103–3138. https://doi.org/10.1007/s10531-019-01827-3.
Angelstam P, Kuuluvainen T (2004) Boreal forest disturbance regimes, successional dynamics and landscape structures: a European perspective. Ecol Bull 51: 117–136.
Belinchón R, Harrison PJ, Mair L, Várkonyi G, Snäll T (2017) Local epiphyte establishment and future metapopulation dynamics in landscapes with different spatiotemporal properties. Ecology 98: 741–750. https://doi.org/10.1002/ecy.1686.
Berglund H, Jonsson BG (2005) Verifying an extinction debt among lichens and fungi in Northern Swedish boreal forests. Conserv Biol 19: 338–348. https://doi.org/10.1111/j.1523-1739.2005.00550.x.
Cáceres MES, Lücking R, Rambold G (2007) Phorophyte specificity and environmental parameters versus stochasticity as determinants for species composition of corticolous crustose lichen communities in the Atlantic rain forest of northeastern Brazil. Mycol Prog 6: 117–136. https://doi.org/10.1007/s11557-007-0532-2.
Cajander AK (1949) Forest types and their significance. Acta For Fenn 56: 1–71. https://doi.org/10.14214/aff.7396.
Chen J, Franklin JF, Spies TA (1993) Contrasting microclimates among clearcut, edge, and interior of old-growth Douglas-fir forest. Agr Forest Meteorol 63: 219–237. https://doi.org/10.1016/0168-1923(93)90061-L.
Duncker PS, Barreiro SM, Hengeveld GM, Lind T, Mason WL, Ambrozy S, Spiecker H (2012) Classification of forest management approaches: a new conceptual framework and its applicability to European forestry. Ecol Soc 17, article id 51. http://doi.org/10.5751/ES-05262-170451.
Ellis CJ, Coppins BJ (2007) 19th century woodland structure controls stand-scale epiphyte diversity in present-day Scotland. Divers Distrib 13: 84–91. https://doi.org/10.1111/j.1366-9516.2006.00310.x.
Ellis CJ (2012) Lichen epiphyte diversity: a species, community and trait-based review. Perspect Plant Ecol 14: 131–152. https://doi.org/10.1016/j.ppees.2011.10.001.
Esseen P, Ehnström B, Ericson L, Sjöberg K (1997) Boreal forests. Ecol Bull 46: 16–47.
Fedrowitz K, Kuusinen M, Snäll T (2012) Metapopulation dynamics and future persistence of epiphytic cyanolichens in a European boreal forest ecosystem. J Appl Ecol 49: 493–502. https://doi.org/10.1111/j.1365-2664.2012.02113.x.
Gauslaa Y, Lie M, Solhaug KA, Ohlson M (2006) Growth and ecophysiological acclimation of the foliose lichen Lobaria pulmonaria in forests with contrasting light climates. Oecologia 147, article id 406. https://doi.org/10.1007/s00442-005-0283-1.
Gauslaa Y, Palmqvist K, Solhaug K, Holien H, Hilmo O, Nybakken L (2007) Growth of epiphytic old forest lichens across climatic and successional gradients. Can J Forest Res 37: 1832–1845. https://doi.org/10.1139/X07-048.
Giordani P, Calatayud C, Stofer S, Seidling W, Granke O, Fischer R (2014) Detecting the nitrogen critical loads on European forests by means of epiphytic lichens. A signal-to-noise evaluation. For Ecol Manag 311: 29–40. https://doi.org/10.1016/j.foreco.2013.05.048.
Grimmond CSB, Robeson SM, Schoof JT (2000) Spatial variability of micro-climatic conditions within a mid-latitude deciduous forest. Clim Res 15: 137–149. https://doi.org/10.3354/cr015137.
Gustafsson L, Hannerz M, Koivula M, Shorohova E, Vanha-Majamaa I, Weslien J (2020) Research on retention forestry in Northern Europe. Ecological Processes 9, article id 3. https://doi.org/10.1186/s13717-019-0208-2.
Hämäläinen K, Junninen K, Halme P, Kouki J (2020) Managing conservation values of protected sites: how to maintain deciduous trees in white-backed woodpecker territories. For Ecol Manag 461, article id 117946. https://doi.org/10.1016/j.foreco.2020.117946.
Hanski I (2000) Extinction debt and species credit in boreal forests: modelling the consequences of different approaches to biodiversity conservation. Ann Zool Fenn 37: 271–280.
Hardenbol AA, Junninen K, Kouki J (2020) A key tree species for forest biodiversity, European aspen (Populus tremula), is rapidly declining in boreal old-growth forest reserves. For Ecol Manag 462, article id 118009. https://doi.org/10.1016/j.foreco.2020.118009.
Hawksworth D, Rose F (1970) Qualitative scale for estimating sulphur dioxide air pollution in England and Wales using epiphytic lichens. Nature 227: 145–148. https://doi.org/10.1038/227145a0.
Hillebrand H (2004) On the generality of the latitudinal diversity gradient. Am Nat 163: 192–211. https://doi.org/10.1086/381004.
Hyvärinen E, Juslén A, Kemppainen E, Uddström A, Liukko U-M (2019) Suomen lajien uhanalaisuus – Punainen kirja 2019. [The 2019 Red List of Finnish Species]. Ympäristöministeriö & Suomen ympäristökeskus, Helsinki.
IUCN (2022) Guidelines for Using the IUCN Red List Categories and Criteria. Version 15. https://www.iucnredlist.org/resources/redlistguidelines. Accessed 9 November 2022.
Johansson V, Ranius T, Snäll T (2012) Epiphyte metapopulation dynamics are explained by species traits, connectivity, and patch dynamics. Ecology 93: 235–241. https://doi.org/10.1890/11-0760.1.
Johansson V, Ranius T, Snäll T (2013a) Epiphyte metapopulation persistence after drastic habitat decline and low tree regeneration: time-lags and effects of conservation actions. J Appl Ecol 50: 414–422. https://doi.org/10.1111/1365-2664.12049.
Johansson V, Snäll T, Ranius T (2013b) Estimates of connectivity reveal non-equilibrium epiphyte occurrence patterns almost 180 years after habitat decline. Oecologia 172: 607–615. https://doi.org/10.1007/s00442-012-2509-3.
Johansson V, Wikström C, Hylander K (2018) Time-lagged lichen extinction in retained buffer strips 16.5 years after clearcutting. Biol Conserv 225: 53–65. https://doi.org/10.1016/j.biocon.2018.06.016.
Joppa LN, Pfaff A (2009) High and far: biases in the location of protected areas. PLoS ONE 4, article id e8273. https://doi.org/10.1371/journal.pone.0008273.
Kivinen S, Koivisto E, Keski-Saari S, Poikolainen L, Tanhuanpää T, Kuzmin A, Viinikka A, Heikkinen RK, Pykälä J, Virkkala R, Vihervaara P, Kumpula T (2020) A keystone species, European aspen (Populus tremula L.), in boreal forests: ecological role, knowledge needs and mapping using remote sensing. For Ecol Manag 462, article id 118008. https://doi.org/10.1016/j.foreco.2020.118008.
Klein J, Thor G, Low M, Sjögren J, Lindberg E, Eggers S (2020) What is good for birds is not always good for lichens: Interactions between forest structure and species richness in managed boreal forests. For Ecol Manag 473, article id 118327. https://doi.org/10.1016/j.foreco.2020.118327.
Komonen A, Tuominen L, Purhonen J, Halme P (2020) Landscape structure influences browsing on a keystone tree species in conservation areas. For Ecol Manag 457: 117724. https://doi.org/10.1016/j.foreco.2019.117724.
Korhonen K, Ahola A, Heikkinen J, Henttonen HM, Hotanen J, Ihalainen A, Melin M, Pitkänen J, Räty M, Sirviö M, Strandström M (2021a) Forests of Finland 2014–2018 and their development 1921–2018. Silva Fenn 55, article id 10662. https://doi.org/10.14214/sf.10662.
Korhonen A, Penttilä R, Siitonen J, Miettinen O, Immonen A, Hamberg L (2021b) Urban forests host rich polypore assemblages in a Nordic metropolitan area. Landscape Urban Plan 215, article id 104222. https://doi.org/10.1016/j.landurbplan.2021.104222.
Kulha N, Pasanen L, Holmström L, De Grandpré L, Gauthier S, Kuuluvainen T, Aakala T (2020) The structure of boreal old-growth forests changes at multiple spatial scales over decades. Landscape Ecol 35: 843–858. https://doi.org/10.1007/s10980-020-00979-w.
Kuuluvainen T (2009) Forest management and biodiversity conservation based on natural ecosystem dynamics in Northern Europe: the complexity challenge. Ambio 38: 309–315. https://doi.org/10.1579/08-A-490.1.
Kuuluvainen T, Lindberg H, Vanha-Majamaa I, Keto-Tokoi P, Punttila P (2019) Low-level retention forestry, certification, and biodiversity: case Finland. Ecol Process 8, article id 47. https://doi.org/10.1186/s13717-019-0198-0.
Kuuluvainen T, Angelstam P, Frelich L, Jõgiste K, Koivula M, Kubota Y, Lafleur B, Macdonald E (2021) Natural disturbance-based forest management: moving beyond retention and continuous-cover forestry. Front For Glob Change 4, article id 629020. https://doi.org/10.3389/ffgc.2021.629020.
Kuusinen M (1996) Epiphyte flora and diversity on basal trunks of six old-growth forest tree species in southern and middle boreal Finland. Lichenologist 28: 443–463. https://doi.org/10.1006/lich.1996.0043.
Lankia H, Wallenius T, Várkonyi G, Kouki J, Snäll T (2012) Forest fire history, aspen and goat willow in a Fennoscandian old-growth landscape: are current population structures a legacy of historical fires? J Veg Sci 23: 1159–1169. https://doi.org/10.1111/j.1654-1103.2012.01426.x.
Larsen JB, Angelstam P, Bauhus J, Carvalho JF, Diaci J, Dobrowolska D, Gazda A, Gustafsson L, Krumm F, Knoke T, Konczal A, Kuuluvainen T, Mason B, Motta R, Pötzelsberger E, Rigling A, Schuck A (2022) Closer-to-Nature Forest Management. From Science to Policy 12. European Forest Institute. https://doi.org/10.36333/fs12.
Lilja S, Wallenius T, Kuuluvainen T (2006) Structure and development of old Picea abies forests in northern boreal Fennoscandia. Écoscience 13: 181–192. https://doi.org/10.2980/i1195-6860-13-2-181.1.
Linder P, Elfving B, Zackrisson O (1997) Stand structure and successional trends in virgin boreal forest reserves in Sweden. For Ecol Manag 98: 17–33. https://doi.org/10.1016/S0378-1127(97)00076-5.
Llewelyn T, Gaya E, Murrell DJ (2020) Are urban communities in successional stasis? A case study on epiphytic lichen communities. Diversity 12: 330. https://doi.org/10.3390/d12090330.
Lõhmus A, Lõhmus P (2010) Epiphyte communities on the trunks of retention trees stabilise in 5 years after timber harvesting, but remain threatened due to tree loss. Biol Conserv 143: 891–898. https://doi.org/10.1016/j.biocon.2009.12.036.
Mace G, Collar NJ, Gaston KJ, Hilton-Taylor C, Akcakaya HR, Leader-Williams N, Milner-Gullard EJ, Stuart SN (2008) Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv Biol 22: 1424–1442. https://doi.org/10.1111/j.1523-1739.2008.01044.x.
Mäkinen H, Hynynen J, Siitonen J, Sievänen R (2006) Predicting the decomposition of Scots pine, Norway spruce and birch stems in Finland. Ecol Appl 16: 1865–1879. https://doi.org/10.1890/1051-0761(2006)016[1865:PTDOSP]2.0.CO;2.
Marmor L, Tõrra T, Saag L, Randlane T (2012) Species richness of epiphytic lichens in coniferous forests: the effect of canopy openness. Ann Bot Fenn 49: 352–358. https://doi.org/10.5735/085.049.0606.
Marmor L, Randlane T, Jüriado I, Saag A (2017) Host tree preferences of red-listed epiphytic lichens in Estonia. Balt For 23: 364–373.
Mönkkönen M, Aakala T, Blattert C, Burgas D, Duflot R, Eyvindson K, Kouki J, Laaksonen T, Punttila P (2022) More wood but less biodiversity in forests in Finland: a historical evaluation. Memoranda Societas Pro Flora et Fauna Fennica 98, Suppl. 2: 1–11.
Myking T, Solberg EJ, Austrheim S, Speed JDM, Bøhler F, Astrup R, Eriksen R (2013) Browsing of sallow (Salix caprea L.) and rowan (Sorbus aucuparia L.) in the context of life history strategies: a literature review. Eur J For Res 132:399409. https://doi.org/10.1007/s10342-013-0684-3.
Nascimbene J, Nimis PL, Ravera S (2013) Evaluating the conservation status of epiphytic lichens of Italy: a red list. Plant Biosyst 147: 898–904. https://doi.org/10.1080/11263504.2012.748101.
Niemelä T, Wallenius T, Kotiranta H (2002) The kelo tree, a vanishing substrate of specified wood-inhabiting fungi. Polish Botanical Journal 47: 91–101.
Nilsson AR, Solhaug KA, Gauslaa Y (2022) The globally threatened epiphytic cyanolichen Erioderma pedicellatum depends on a rare combination of habitat factors. Lichenologist 54: 123–136. https://doi.org/10.1017/S002428292200007X.
Nirhamo A, Pykälä J, Halme P, Komonen A (2021) Lichen communities on Populus tremula are affected by the density of Picea abies. Appl Veg Sci 24: e12584. https://doi.org/10.1111/avsc.12584.
Palmqvist K (2000) Carbon economy in lichens. New Phytol 148: 11–36. https://doi.org/10.1046/j.1469-8137.2000.00732.x.
Perhans K, Appelgren L, Jonsson F, Nordin U, Gustafsson L (2009) Retention patches as potential refugia for bryophytes and lichens in managed forest landscapes. Biol Conserv 142: 1125–1133. https://doi.org/10.1016/j.biocon.2008.12.033.
Petersson L, Lariviere D, Holmström E, Fritz Ö, Felton A (2022) Conifer tree species and age as drivers of epiphytic lichen communities in northern European production forests. Lichenologist 54: 213–225. https://doi.org/10.1017/S0024282922000172.
Pykälä J, Heikkinen RK, Toivonen H, Jääskeläinen K (2006) Importance of Forest Act habitats for epiphytic lichens in Finnish managed forests. For Ecol Manag 223: 84–92. https://doi.org/10.1016/j.foreco.2005.10.059.
Pykälä J, Jääskeläinen K, Rämä H, Launis A, Vitikainen O, Puolasmaa A (2019). Jäkälät. [Lichens]. In: Hyvärinen E, Juslén A, Kemppainen E, Uddström A, Liukko U-M (eds) Suomen lajien uhanalaisuus – Punainen kirja 2019. Ympäristöministeriö & Suomen ympäristökeskus, Helsinki, pp 263–312.
Pykälä J, Velmala S, Ahti T, Myllys L (2022) Lichens. In: FinBIF 2022: the FinBIF checklist of Finnish species 2021. Finnish Biodiversity Information Facility, Finnish Museum of Natural History, Helsinki.
Rassi P, Hyvärinen E, Juslén A, Mannerkoski I (2010) Suomen lajien uhanalaisuus – Punainen kirja 2010. [The 2010 Red List of Finnish Species]. Ympäristöministeriö & Suomen ympäristökeskus, Helsinki.
Rosabal D, Burgaz AR, Reyes OJ (2013) Substrate preferences and phorophyte specificity of corticolous lichens on five tree species of the montane rainforest of Gran Piedra, Santiago de Cuba. The Bryologist 116: 113–121. https://doi.org/10.1639/0007-2745-116.2.113.
Rosenvald R, Lõhmus P, Rannap R, Remm L, Rosenvald K, Runnel K, Lõhmus A (2019) Assessing long-term effectiveness of green-tree retention. For Ecol Manag 448: 543–548. https://doi.org/10.1016/j.foreco.2019.06.034.
Rubio-Salcedo M, Psomas A, Prieto M, Zimmermann NE, Martínez I (2017) Case study of the implications of climate change for lichen diversity and distributions. Biodivers Conserv 26: 1121–1141. https://doi.org/10.1007/s10531-016-1289-1.
Santaniello F, Djupström LB, Ranius T, Weslien J, Rudolphi J, Thor G (2017) Large proportion of wood dependent lichens in boreal pine forest are confined to old hard wood. Biodivers Conserv 26: 1295–1310. https://doi.org/10.1007/s10531-017-1301-4.
Schmitt CK, Slack NG (1990) Host specificity of epiphytic lichens and bryophytes: a comparison of the Adirondack mountains (New York) and the Southern Blue Ridge Mountains (North Carolina). The Bryologist 93: 257–274. https://doi.org/10.2307/3243509.
Siitonen J, Martikainen P, Punttila P, Rauh J (2000) Coarse woody debris and stand characteristics in mature managed and old-growth boreal mesic forests in southern Finland. For Ecol Manag 128: 211–225. https://doi.org/10.1016/S0378-1127(99)00148-6.
Snäll T, Pennanen J, Kivistö L, Hanski I (2005) Modelling epiphyte metapopulation dynamics in a dynamic forest landscape. Oikos 109: 209–222. https://doi.org/10.1111/j.0030-1299.2005.13616.x.
Svensson M, Johansson P, Thor G (2005) Lichens of wooden barns and Pinus sylvestris snags in Dalarna, Sweden. Ann Bot Fenn 42: 351–363.
Tagirdzhanova G, Stepanchikova IS, Himelbrant DE, Vyatkina MP, Dyomina AV, Dirksen VG, Scheidegger C (2019) Distribution and assessment of the conservation status of Erioderma pedicellatum in Asia. Lichenologist 51: 575–585. https://doi.org/10.1017/S0024282919000380.
Terho M, Hallaksela A-M (2008) Decay characteristics of hazardous Tilia, Betula, and Acer trees felled by municipal urban tree managers in the Helsinki City Area. Forestry 81: 151–159. https://doi.org/10.1093/forestry/cpn002.
Tikkanen O, Martikainen P, Hyvärinen E, Junninen K, Kouki J (2006) Red-listed boreal forest species of Finland: associations with forest structure, tree species, and decaying wood. Ann Zool Fenn 43: 373–383.
Total of 73 references.
Send to email

