Indicators of ancient forests in nutrient-deficient pine habitats
Kowalska A., Matuszkiewicz J. M., Solon J., Kozłowska A. (2017). Indicators of ancient forests in nutrient-deficient pine habitats. Silva Fennica vol. 51 no. 1 article id 1684. https://doi.org/10.14214/sf.1684
Highlights
- Distinct groups of species with a preference for ancient pine and mixed oak-pine forests can be determined
- The ancient forest indicator composition in pine habitats differs remarkably from ancient forest indicators in deciduous forests
- Dispersal-related traits significantly distinguish ancient forest indicators from other species found in nutrient-poor forest habitats.
Abstract
Pine forests are common in many European regions. Nonetheless, there are only a few studies on regeneration of plant species populations in nutrient-deficient pine habitats. Ancient temperate forests are perceived to be particularly important objects of environmental conservation, due to their ability to sustain a considerable number of rare and vulnerable species. In this paper, we present indicator species of ancient pine and mixed oak-pine forests, together with their trait profiles. Phytosociological relevés were collected from mature stands in the Masuria and Kurpie regions of central Poland. Forest persistence was determined on the basis of historical maps, with the data set divided into three categories. The indicator value of species was evaluated using Tichý and Chytrý’s phi coefficient. Functional response traits of indicator species were identified. Distinct groups of species with a preference for ancient forests can be determined. The dispersal-related traits significantly distinguish ancient forest indicators from other species found in nutrient-poor forest habitats. Since the low potential for long-distance dispersal hinders the establishment of new plant populations in isolated stands, we stress the need to avoid ancient forest clearance and fragmentation of woodland; afforestation should be located in the vicinity of ancient stands. Moreover, as recent forests have turned out to support several rare plant species, to maintain phytodiversity on a landscape level a mixture of ancient and recent forests, both managed and strictly protected, is needed.
Keywords
forest continuity;
central Poland;
life-history traits;
mixed oak-pine forests;
phi coefficient;
pine forests
-
Kowalska,
Institute of Geography and Spatial Organization, Polish Academy of Science, Twarda 51/55, 00-818 Warsaw, Poland
E-mail
aniak@twarda.pan.pl

- Matuszkiewicz, Institute of Geography and Spatial Organization, Polish Academy of Science, Twarda 51/55, 00-818 Warsaw, Poland E-mail jan.mat@twarda.pan.pl
- Solon, Institute of Geography and Spatial Organization, Polish Academy of Science, Twarda 51/55, 00-818 Warsaw, Poland E-mail j.solon@twarda.pan.pl
- Kozłowska, Institute of Geography and Spatial Organization, Polish Academy of Science, Twarda 51/55, 00-818 Warsaw, Poland E-mail a.kozl@twarda.pan.pl
Received 12 August 2016 Accepted 11 January 2017 Published 17 January 2017
Views 179454
Available at https://doi.org/10.14214/sf.1684 | Download PDF

Supplementary Files
1 Introduction
Forest cover in Europe has been constantly increasing within the last few decades (Forest Europe, UNECE, FAO 2011), and is now ~45% of the European land area. Therein, coniferous forests comprise nearly 50% of the area, while deciduous forests and mixed forests ~25% each. Most are semi-natural communities (~70%). Forests undisturbed by man constitute only 26% of their range. Both forest classes can be partly identified with ancient forests – plant communities with long habitat continuity. This term refers to forests that have a persistence of at least some hundreds of years (Rackham 1980). The threshold date depends on the availability of historical maps and other materials documenting their origin. In Europe, such materials date back to the 17th century (England – Peterken 1977), and to the 18th and 19th centuries (other regions: Belgium, Poland, Denmark, Germany, Sweden etc. – Hermy and Stieperaere 1981; Dzwonko and Loster 1988; Petersen 1994; Wulf 1997; Brunet and von Oheimb 1998).
Ancient temperate forests are perceived to be particularly important objects of environmental conservation, due to their ability to sustain a considerable number of rare and vulnerable species (Goldberg et al. 2007; Kimberley et al. 2013). Their floral composition differs from recent afforestations (Graae and Heskjær 1997; Graae et al. 2003; Verheyen et al. 2003a), because the natural regeneration of typical forest plant species populations is very slow, and may take many centuries (Faliński 1986). Such species are called ancient forest indicators (Peterken 1974; Rose 1999). Their distribution has proved to be limited by dispersal ability and seed longevity (Jankowska-Błaszczuk and Grubb 1997; Bekker et al. 1998a; Thompson et al. 1998), as well as by phytocoenotic (produced by other plants) and soil conditions (Eriksson 1995; Flinn and Vellend 2005; Hermy and Verheyen 2007). In recent stands, especially post-agricultural, cultivation has brought about a significant transformation of their soil environment, mainly in changes of water balance, soil reaction and biochemical components. The main effects lie in the plough level, which constrains the biological activity of the soil and the organic-matter cycle, higher pH values in the topsoil, higher P- and N-content, lower C-content and a lack of typical forest soil fauna (Bellemare et al. 2002; Falkengren-Grerup et al. 2006).
Ancient forest indicators have specific traits that clearly distinguish them from other forest species. They are usually small perennials with heavy seeds; shade-tolerant, not favoured by intensive disturbance regimes and high nutrient levels (Kimberley et al. 2013). Therefore, they can indicate ancient forests (Rolstad et al. 2002) when historical maps are lacking, or can help to assess forest diversity (Nordén and Appelqvist 2001; Matuszkiewicz et al. 2013a; Schmidt et al. 2014). This knowledge could be useful in the formulation of protection plans for forests, or establishment of new nature reserves.
Lists of ancient forest indicator species have been developed in several regions of Europe (Wulf 1997; Honnay et al. 1998; Rose 1999; Dzwonko and Loster 2001). The authors suggest that they should be interpreted with caution, as the association with ancient forests can differ across regions with a variety of geological substratum, soil and climatic conditions, as well as species’ range. Therefore, regarding the composition of ancient forest indicator species, habitat conditions should differentiate between deciduous, coniferous and mixed forest types. Some studies have already confirmed these relationships (Dzwonko 2001a; Wulf and Heinken 2008; Schmidt et al. 2014).
Pinus sylvestris L. is one of the species most often used for reforestation and has a large range, extending from Arctic latitudes in Norway to southern mountain areas of Spain (Marcos et al. 2007; Distribution map... 2009). Pine forests account for the majority of forest sites in large parts of the northern Central European lowlands (Heinken 2008; Reinecke et al. 2014). They are also the most common plantations in Poland (~60% of all forests – Forestry. Statistical Yearbook of the Republic of Poland 2013). The colonization of these habitats is a long process, mainly because of their thick, acidic, slow-to-decompose needle litter, which hampers recruitment and growth of typical forest species (Kuiters and Denneman 1987; Ericksson 1995; Dzwonko 2001a). Surprisingly, considering the high proportion of coniferous forests in Europe, there are only a few studies on species regeneration in such nutrient-deficient sites (Summers et al. 1999; Orczewska and Fernes 2011; Matuszkiewicz et al. 2013b). Moreover, the existing lists of ancient forest indicators (Hermy et al. 1999; Dzwonko and Loster 2001) are mainly composed of rich, broadleaved forest habitats species, with hardly any species characteristic of coniferous forests. In this context, it is very important and useful in forest protection plans to determine indicator species of ancient forests in acidic forest habitats and especially their trait profiles. This knowledge should be universal for the entire area where such types of forests are distributed, because it allows comparability among studies with different species pools (Verheyen et al. 2003b).
We addressed the following questions:
- What are the indicator species of ancient pine and mixed oak-pine forests?
- How distinct are the trait profiles of ancient pine and oak-pine forest indicators (AFIs) from that of other plant species (non-AFIs)?
- What recommendations can be put forward for pine and mixed oak-pine forest management to enhance phytodiversity?
2 Methods
2.1 Study area
The study was conducted in central Poland, in the regions of Masuria and Kurpie, located between 53°10´N and 53°67´N, and 20°53´E and 21°69´E (Fig. 1). The study area encompassed 2843.7 km2. Forests cover about 47% of the area. The majority (~75%) are associated with pine and mixed oak-pine forest habitats on dunes and outwash fields. The more fertile habitats, mainly on moraines, lobes and hills, are mostly deforested. Most pine and oak-pine stands represent recent forest communities growing on former agricultural land. Ancient forests, defined as forests that have existed continuously since at least 1800, without a trace of a plough level in the soil, constitute only 40% of the forest stands.
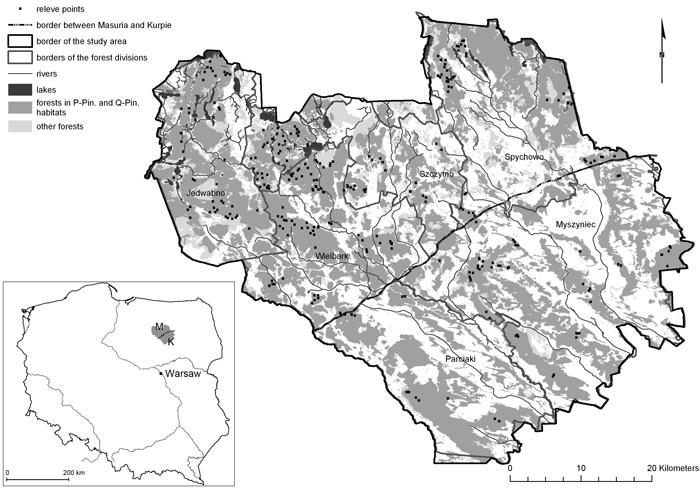
Fig. 1. Location of the study area in Poland and distribution of the relevés in Peucedano-Pinetum and Querco roboris-Pinetum habitats. View larger in new window/tab.
2.2 Data collection
Peucedano-Pinetum W. MAT. (1962) 1973 pine forests and Querco roboris-Pinetum (W.MAT. 1981) J.MAT. 1988 mixed oak-pine forests were surveyed in summer 2010 and 2011. Both forest associations belong to the Vaccinio-Piceetea class, but in Querco roboris-Pinetum numerous species of Querco-Fagetea class are constantly observed (Table S1) and Quercus robur L. co-dominates or dominates over Pinus sylvestris in the stand (Matuszkiewicz 2001). Sampling points were selected in present, mature stands between 62 and 190 years old, free from the kinds of heavy disturbance resulting from silvicultural practices (e.g. with tree or shrub species extraneous to the habitat types, post-felling communities with big canopy gaps etc). They were located in the forest interior at a minimum distance between recent sampling plots and ancient forest stands of at least 200 m (much further than most studied ancient forest species are able to migrate during 200 years – see Orczewska and Fernes 2011, p. 79) (Fig. 2). We collected 296 phytosociological relevés in accordance to the Braun-Blanquet methodology (1964; Dzwonko 2007). All tree, shrub, herb, moss and lichen species were recorded within plots set at 400 m2 in the most uniform forest patches (without hills or ground lowering etc.). The horizontal structure of vegetation was described with the abundance scale proposed by Braun-Blanquet (1964), which takes into account the relationship between a number of individuals and their cover (the scale consists of six degrees: 5 – “the species covers 75–100% of the area”, 4 – “50–75%”, 3 – “25–50%”, 2 – “5–25%”, 1 – “below 5%”, + – “the species is barely represented”). The vertical structure was characterised as a percentage of the forest layers cover. The origin of each forest stand was ascertained with the help of six historical topographical maps, the oldest dating back to the 1800s (Matuszkiewicz et al. 2013c). On the basis of cartographic analysis, the persistence of forest stands was determined and the data set was divided into three categories: ancient forest (57 relevés – P-Pin., 30 – Q-Pin.), and two groups of recent forests with different regeneration times (145 relevés – P-Pin., 64 – Q-Pin. together) (Table 1). Previous agricultural use was proved in the field, by identification of the plough level, based on visual evaluation of soil profiles. Description of the soil profile morphology and diagnosis of soil and humus types also verified the selection of forest habitats. The chemical analysis of soil samples, collected from each horizon of the 200 soil profiles (randomly selected from the studied stands), showed that the shorter the forest persistence, the lower the content of organic carbon and total nitrogen, and the higher the value of the C:N ratio and pH value (C and N content was determined using VarioMax CNS Element analyser, pH – by potentiomertic measurement in H2O – Matuszkiewicz et al. 2013a). These findings indicated a rather limited use of fertilizers during soil cultivation, but a significant impact by former land use. Soils in the studied mixed oak-pine forests showed better properties and higher biological activity enhancing their regeneration than soils under pine forests.
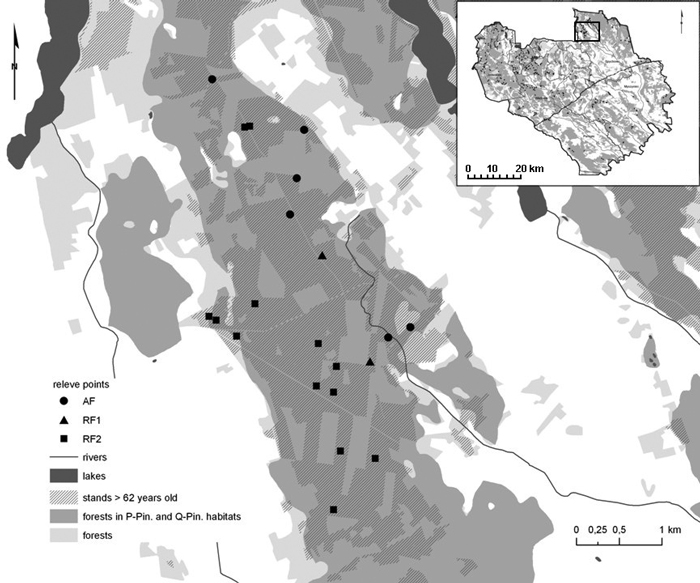
Fig. 2. Section of the study area with the location of the relevés (AF – ancient forests; RF1, RF2 – recent forests; Table 1).
| Table 1. Forest categories distinguished after cartographic analysis and field studies. | |
| Forest category | |
| AF | ancient forests defined as forests that have existed continuously since at least 1800; forest origin ascertained with the help of maps from 1800–1830; without a post-agricultural horizon in the soil |
| RF1 | recent, post-agricultural forest regenerated over 200 years; forest origin ascertained with the help of maps from 1800–1830; with a distinct post-agricultural horizon in the soil |
| RF2 | recent, post-agricultural forest regenerated in the last 80–180-year period; forest origin ascertained with the help of maps from 1885, 1928, 1950; with a post-agricultural horizon in the soil |
2.3 Data analysis
To determine indicator species of ancient forests, a statistical measure of fidelity, Tichý and Chytrý’s (2006) phi coefficient, was used. It was applied to presence-absence data and was adjusted to correct unequal sample sizes among groups (McCune and Mefford 2011), which is important in our case. For each species of herb, moss and lichen present in at least 10% of the forest plots in any forest category, the phi coefficient was computed (Lists of all the species recorded, with their frequency and mean species number by relevé, are presented in Supplementary file – Table S1). The phi coefficients range from –1 (perfect negative indication) to 1 (perfect positive indication). The final indicator value of a species equalled the maximal indicator value from all categories. The randomization technique (Monte Carlo test) was used to evaluate the statistical significance of the index of each species. All analyses were performed using PC-ORD (version 6, MjM Software, Gleneden Beach, Oregon, USA).
Nine functional response traits of species were used, representing those life-history attributes considered most likely to differ between AFIs and non-AFIs in other studies of ancient vs. recent forests (e.g. Kimberley et al. 2013; Kelemen et al. 2014) (Table 2). This set comprised various dispersal-related traits and competitive and shade-tolerant strategies, e.g. species-specific leaf area (SLA) that has been associated with light conditions and nutrient availability (a high value is thought to indicate shade tolerance as well as productive, human-modified habitats – Pérez-Harguindeguy et al. 2013). The trait data were mainly obtained from the LEDA trait-base (Kleyer et al. 2008) and other reference materials (for missing data on dispersal type, ecological affinity and maximum height – Müller-Schneider 1983; Düll and Kutzelnigg 1986; Lindacher 1995; Witkowska-Żuk 2008).
| Table 2. Variables included in the analyses (for species recorded in both forest types). | |||
| Trait | Description | Variable type | Number of species with trait data available (max. = 50) |
| Max. height | Maximum height of plant individual in cm | continuous | 49 |
| Growth form | Two classes: graminoids and herbs | categorical | 50 |
| Life span | Perennial/annual | categorical | 50 |
| SLA | Specific leaf area (mm2 mg–1) | continuous | 48 |
| Seed longevity index | Proportion (%) of short- and long-term persistent records on total (Bekker et al. 1998b) | continuous | 35 |
| Seed weight | Weight of 1000 dried seeds (g) | continuous | 39 |
| Seed number | Seed number per plant | continuous | 43 |
| Dispersal type | Two categories: long- and short-distance dispersal | categorical | 50 |
| EA | Ecological affinity: forest or open habitats | categorical | 50 |
Mean values of continuous variables were compared across AFIs and non-AFIs using the Mann-Whitney U-test. Traits of categorical types were compared using Fisher’s exact probability test. The statistical analyses were carried out using the Statistica 7.1 package.
3 Results
3.1 Indicators of ancient forests
Higher mean species numbers in the relevé were observed in ancient forests (Table S1), but the differences were no significant, especially in pine forests.
Twelve species were found to be the most indicative of ancient forests in pine forest habitats (Table 3). There are ten herbs (including Athyrium filix-femina (L.) Roth, Calamagrostis arundinacea (L.) Roth, Calluna vulgaris (L.) Hull) and two moss species (Hylocomium splendens (Hedw.) Schimp., Pohlia nutans (Hedw.) Lindb.). Five plants (Convalaria majalis L., Luzula pilosa (L.) Willd., Molinia caerulea (L.) Moench, Scorzonera humilis L., Trientalis europaea L.) also show a significant preference for ancient mixed oak-pine forests (Table 4). Two herbs (C. vulgaris, Vaccinium vitis-idaea L.) are indicators of the oldest recent mixed oak-pine forests. Moreover, there are four species (Maianthemum bifolium (L.) DC, Polygonatum odoratum (Mill.) Druce, Pteridium aquilinum (L.) Kuhn, Sciuro-hypnum oedipodium (Mitt.) Ignatov & Huttunen), rarely observed in pine forests, which indicate ancient mixed oak-pine forests.
| Table 3. Percentage frequency of species and their indicator values computed using the phi coefficient for three pine forest categories: AF – ancient forests; RF1, RF2 – recent forests with different persistence; p – statistical significance; bold indicates max. values with the significance of the test between categories p ≤ 0.05. | |||||
| Species name | AF | RF1 | RF2 | p | |
| No. of relevés | 57 | 28 | 117 | ||
| frequency (%) phi value | |||||
| herbs | |||||
| Agrostis capillaris | 8.8–0.025 | 3.6–0.124 | 12.00.082 | 0.476 | |
| Anthoxanthum odoratum | 1.8–0.321 | 14.3–0.096 | 32.50.354 | 0.006 | |
| Athyrium filix-femina** | 15.80.282 | 3.6–0.047 | 0.9–0.188 | 0.002 | |
| Calamagrostis arundinacea | 38.60.244 | 17.9–0.065 | 16.2–0.166 | 0.010 | |
| Calamagrostis epigejos | 7.00.030 | 10.70.102 | 4.3–0.074 | 0.351 | |
| Calluna vulgaris | 98.20.452 | 78.60.145 | 47.9–0.493 | <0.001 | |
| Chimaphila umbellata | 5.3–0.256 | 3.6–0.251 | 32.50.378 | <0.001 | |
| Convallaria majalis** | 43.90.467 | 17.90.022 | 2.6–0.359 | <0.001 | |
| Deschampsia flexuosa | 77.2–0.145 | 78.6–0.097 | 90.60.159 | 0.076 | |
| Dryopteris carthusiana** | 24.6–0.146 | 28.6–0.076 | 41.90.163 | 0.092 | |
| Festuca ovina | 19.3–0.232 | 46.40.116 | 41.90.137 | 0.134 | |
| Hieracium lachenalii | 3.5–0.100 | 0.0–0.175 | 11.10.185 | 0.066 | |
| Luzula pilosa** | 42.10.251 | 25.0–0.003 | 17.1–0.199 | 0.007 | |
| Lycopodium annotinum* | 10.5–0.046 | 10.7–0.036 | 14.5–0.057 | 0.664 | |
| Lycopodium clavatum | 5.3–0.238 | 14.3–0.071 | 27.40.255 | 0.008 | |
| Melampyrum pratense** | 96.50.135 | 96.40.117 | 86.3–0.189 | 0.127 | |
| Molinia caerulea | 19.30.283 | 7.1–0.006 | 1.7–0.207 | 0.001 | |
| Monotropa hypopitys | 1.8–0.169 | 10.70.034 | 12.00.133 | 0.168 | |
| Peucedanum oreoselinum | 15.80.141 | 7.1–0.044 | 6.8–0.092 | 0.189 | |
| Pyrola chlorantha | 0.0–0.199 | 3.6–0.094 | 12.80.250 | 0.005 | |
| Rumex acetosella | 3.5–0.373 | 28.6–0.002 | 41.00.336 | <0.001 | |
| Scorzonera humilis | 29.80.349 | 0.0–0.225 | 6.0–0.183 | <0.001 | |
| Solidago virgaurea* | 10.50.003 | 3.6–0.131 | 12.00.060 | 0.733 | |
| Trientalis europaea* | 33.30.342 | 7.1–0.118 | 6.8–0.216 | <0.001 | |
| Vaccinium myrtillus** | 100.00.097 | 96.4–0.058 | 97.4–0.052 | 0.294 | |
| Vaccinium uliginosum | 14.00.313 | 0.0–0.126 | 0.0–0.183 | <0.001 | |
| Vaccinium vitis-idaea** | 100.00.331 | 92.90.179 | 68.4–0.423 | <0.001 | |
| mosses and lichens | |||||
| Cladonia arbuscula | 24.6–0.228 | 46.40.054 | 48.70.165 | 0.079 | |
| Cladonia furcata | 22.8–0.037 | 35.70.128 | 23.9–0.034 | 0.179 | |
| Cladonia rangiferina | 22.80.033 | 14.3–0.090 | 21.40.016 | 0.901 | |
| Dicranum polysetum | 98.20.011 | 100.00.088 | 97.4–0.052 | 0.723 | |
| Dicranum scoparium | 38.60.112 | 28.6–0.025 | 27.4–0.080 | 0.222 | |
| Hylocomium splendens | 91.20.300 | 71.40.008 | 60.7–0.268 | 0.001 | |
| Leucobryum glaucum | 22.80.024 | 28.60.095 | 18.8–0.066 | 0.408 | |
| Pohlia nutans | 17.50.352 | 0.0–0.141 | 0.0–0.206 | <0.001 | |
| Polytrichum formosum | 43.90.105 | 28.6–0.087 | 34.2–0.045 | 0.306 | |
| Polytrichum juniperinum | 3.5–0.352 | 21.4–0.067 | 39.30.357 | <0.001 | |
| Ptilium crista-castrensis | 28.10.005 | 25.0–0.034 | 28.2–0.012 | 0.977 | |
| * – ancient forest species according to Dzwonko and Loster (2001) ** – according to Hermy et al. (1999) and Dzwonko and Loster (2001) | |||||
| Table 4. Percentage frequency of species and their indicator values computed using the phi coefficient for three mixed oak-pine forest categories: AF – ancient forests; RF1, RF2 – recent forests with different persistence; p – statistical significance; bold indicates max. values with the significance of the test between categories p ≤ 0.05. | |||||
| Species name | AF | RF1 | RF2 | p | |
| No. of relevés | 30 | 13 | 51 | ||
| frequency (%) phi value | |||||
| herbs | |||||
| Agrostis capillaris | 23.30.116 | 15.4–0.024 | 13.7–0.087 | 0.516 | |
| Anthoxanthum odoratum | 10.0–0.301 | 15.4–0.178 | 45.10.376 | 0.003 | |
| Athyrium filix-femina** | 33.30.225 | 30.80.138 | 9.8–0.248 | 0.120 | |
| Calamagrostis arundinacea | 73.30.194 | 61.50.022 | 51.0–0.184 | 0.196 | |
| Calluna vulgaris | 50.00.104 | 69.20.292 | 31.4–0.231 | 0.039 | |
| Carex digitata** | 16.70.166 | 7.7–0.036 | 5.9–0.120 | 0.237 | |
| Chamaenerion angustifolium | 16.70.135 | 7.7–0.054 | 7.8–0.089 | 0.380 | |
| Chimaphila umbellata | 3.3–0.293 | 23.10.039 | 29.40.259 | 0.039 | |
| Convallaria majalis** | 76.70.544 | 30.8–0.086 | 17.6–0.426 | <0.001 | |
| Cytisus scoparius | 3.3–0.254 | 7.7–0.143 | 27.50.328 | 0.014 | |
| Danthonia decumbens | 10.0–0.014 | 7.7–0.054 | 11.80.038 | 1.000 | |
| Deschampsia flexuosa | 83.3–0.013 | 76.9–0.102 | 86.30.061 | 0.805 | |
| Diphasiastrum complanatum | 3.3–0.087 | 15.40.177 | 5.9–0.021 | 0.294 | |
| Dryopteris carthusiana** | 70.00.059 | 46.2–0.223 | 68.60.058 | 0.764 | |
| Dryopteris filix-mas | 13.30.088 | 0.0–0.200 | 9.80.008 | 0.713 | |
| Festuca ovina | 26.70.072 | 23.10.010 | 19.6–0.066 | 0.826 | |
| Fragaria vesca | 26.70.072 | 15.4–0.094 | 21.6–0.019 | 0.828 | |
| Galeopsis pubescens | 10.00.194 | 7.70.086 | 0.0–0.182 | 0.277 | |
| Galium mollugo | 10.0–0.146 | 7.7–0.156 | 11.10.216 | 0.108 | |
| Genista tinctoria | 10.00.194 | 0.0–0.130 | 2.0–0.105 | 0.269 | |
| Hieracium lachenalii | 10.0–0.077 | 7.7–0.102 | 17.60.121 | 0.604 | |
| Holcus mollis | 0.0–0.213 | 0.0–0.188 | 15.70.332 | 0.021 | |
| Luzula pilosa** | 90.00.314 | 76.90.094 | 54.9–0.342 | 0.008 | |
| Lycopodium annotinum* | 26.7–0.047 | 23.1–0.082 | 33.30.081 | 0.759 | |
| Lycopodium clavatum | 0.0–0.328 | 30.80.170 | 25.50.216 | 0.105 | |
| Maianthemum bifolium** | 53.30.317 | 23.1–0.106 | 21.6–0.221 | 0.019 | |
| Melampyrum pratense** | 83.3–0.135 | 100.00.212 | 90.20.027 | 0.230 | |
| Moehringia trinervia | 13.30.012 | 0.0–0.234 | 15.70.095 | 0.585 | |
| Molinia caerulea | 26.70.255 | 7.7–0.102 | 7.8–0.166 | 0.055 | |
| Mycelis muralis* | 20.00.178 | 7.7–0.071 | 7.8–0.116 | 0.309 | |
| Orthilia secunda* | 16.70.013 | 0.0–0.265 | 19.60.107 | 0.490 | |
| Oxalis acetosella** | 53.30.198 | 38.5–0.010 | 31.4–0.166 | 0.154 | |
| Peucedanum oreoselinum | 26.70.227 | 15.40.008 | 7.8–0.189 | 0.062 | |
| Poa augustifolia | 10.00.067 | 0.0–0.175 | 7.80.016 | 0.856 | |
| Polygonatum odoratum* | 33.30.384 | 7.7–0.092 | 3.9–0.042 | 0.003 | |
| Potentilla erecta | 10.00.194 | 0.0–0.130 | 2.0–0.105 | 0.263 | |
| Pteridium aquilinum** | 46.70.313 | 30.80.051 | 13.7–0.283 | 0.030 | |
| Rubus saxatilis | 26.70.091 | 38.50.217 | 13.7–0.180 | 0.154 | |
| Rumex acetosella | 13.3–0.194 | 7.7–0.238 | 37.30.301 | 0.024 | |
| Scorzonera humilis | 20.00.282 | 7.7–0.016 | 2.0–0.212 | 0.029 | |
| Solidago virgaurea* | 20.00.149 | 23.10.158 | 5.9–0.194 | 0.368 | |
| Trientalis europaea* | 83.30.282 | 69.20.062 | 51.0–0.288 | 0.026 | |
| Vaccinium vitis-idaea** | 86.70.150 | 100.00.323 | 66.7–0.299 | 0.028 | |
| Veronica officinalis | 6.7–0.161 | 7.7–0.116 | 21.60.213 | 0.183 | |
| Viola reichenbachiana** | 6.70.041 | 23.10.334 | 0.0–0.205 | 0.023 | |
| Viola riviniana* | 16.70.201 | 7.7–0.016 | 3.9–0.154 | 0.191 | |
| mosses and lichens | |||||
| Aulacomnium palustre | 10.00.143 | 0.0–0.146 | 3.90.061 | 0.369 | |
| Cladonia arbuscula | 0.0–0.213 | 15.40.125 | 11.80.131 | 0.345 | |
| Dicranum polysetum | 83.30.110 | 69.2–0.093 | 74.50.052 | 0.455 | |
| Dicranum scoparium | 26.70.035 | 23.1–0.018 | 23.5–0.022 | 0.946 | |
| Hylocomium splendens | 90.0–0.037 | 84.6–0.125 | 94.10.091 | 0.679 | |
| Plagiomnium affine | 23.30.116 | 7.7–0.143 | 15.7–0.036 | 0.507 | |
| Pleurozium schreberi | 100.00.073 | 100.00.064 | 98.0–0.115 | 1.000 | |
| Pohlia nutans | 10.00.037 | 23.10.247 | 3.9–0.154 | 0.084 | |
| Polytrichum commune | 3.3–0.061 | 15.40.208 | 3.9–0.061 | 0.219 | |
| Polytrichum formosum | 70.00.248 | 38.5–0.150 | 45.1–0.146 | 0.071 | |
| Polytrichum juniperinum | 13.3–0.068 | 0.0–0.275 | 23.50.193 | 0.135 | |
| Ptilium crista-castrensis | 40.0–0.080 | 38.5–0.080 | 51.00.109 | 0.460 | |
| Sciuro-hypnum oedipodium | 23.30.320 | 7.7–0.036 | 2.0–0.233 | 0.026 | |
| * – ancient forest species according to Dzwonko and Loster (2001) ** – according to Hermy et al. (1999) and Dzwonko and Loster (2001) | |||||
3.2 Species traits
Most of the analysed traits do not show significant differences across AFIs and non-AFIs in both forest types. Both species groups are dominated by perennial forest herbs (Table 5). Some annual species belong to non-AFIs, but these are rare. There are also more graminoids among non-AFIs, but they are still less frequent than herbs. Moreover, species characteristic of non-forest habitats are more abundant among non-AFIs (especially in pine forests), but this difference is insignificant (Table 5). There is no remarkable difference in maximal plant height (Table 6). AFIs and non-AFIs do not differ significantly in seed bank persistence. Species with transient seed bank dominate in both groups, but they also consist of plants with a persistent seed bank. Specific leaf area does not clearly distinguish the two species groups either. Interestingly, in pine forests AFIs have lower SLA values than non-AFIs, while in mixed oak-pine forests, in contrast, their SLA values are higher than for non-AFIs. The only trait variables that distinctly discriminate between AFIs and non-AFIs in both forest types are dispersal type and seed number. AFIs are mostly short-distance dispersing species with a small number of seeds. Their seeds are also heavier, although this difference is significant only for AFIs in mixed oak-pine forests (Table 6).
| Table 5. Categorical plant trait variables in pine and mixed oak-pine forests; p value from Fisher’s exact test; bold indicates the significant differences. | |||||||
| Trait | Category | Number of species (pine forest, n = 202) | p value | Number of species (mixed oak-pine forest, n = 84) | p value | ||
| AFI | non-AFI | AFI | non-AFI | ||||
| Growth form | graminoids | 2 | 5 | 0.509 | 1 | 9 | 0.436 |
| herbs | 8 | 13 | 7 | 29 | |||
| Life span | perennial | 10 | 17 | 0.643 | 8 | 35 | 0.556 |
| annual | 0 | 1 | 0 | 3 | |||
| Dispersal type | long-distance | 3 | 18 | 0.037 | 3 | 30 | 0.031 |
| short-distance | 7 | 0 | 5 | 8 | |||
| Ecological affinity | forest | 8 | 8 | 0.076 | 7 | 24 | 0.181 |
| open habitat | 2 | 10 | 1 | 14 | |||
| Table 6. Plant trait variables of continuous type in pine and mixed oak-pine forests; p value from Mann-Whitney U-test; SD – standard deviation; bold indicates the significant differences. | |||||||
| Trait | value | Pine forest (n = 202) | Mixed oak-pine forest (n = 84) | ||||
| AFI | non-AFI | p value | AFI | non-AFI | p value | ||
| Maximum plant height (cm) | Mean (SD) | 61 (36.9) | 60 (43.8) | 0.801 | 62 (60.8) | 59 (35.1) | 0.222 |
| Specific leaf area (mm2 mg–1) | Mean (SD) | 23 (15.4) | 29 (16.9) | 0.206 | 33 (12.1) | 27 (15.5) | 0.167 |
| Seed bank longevity index (ratio) | Mean (SD) | 0.24 (0.27) | 0.22 (0.25) | 0.546 | 0.15 (0.20) | 0.25 (0.23) | 0.213 |
| Seed weight (g) | Mean (SD) | 2.8 (5.86) | 1.01 (1.77) | 0.201 | 9.1 (10.64) | 1.4 (2.09) | 0.011 |
| Seed number | Mean (SD) | 295.9 (410.1) | 1 955 502.6 (7 311 917.7) | 0.044 | 204 (408.3) | 1 113 042.2 (4 902 340.2) | 0.010 |
4 Discussion
4.1 Ancient pine and mixed oak-pine forest indicators
Species with a strong affinity for ancient forests constitute an important part of the composition of the understorey in the studied pine and mixed oak-pine forests, though their number is much lower than in deciduous forests on base-rich soils (Heinken 1998). There are no species that occurred exclusively in ancient stands, but we can distinguish distinct groups of species with a preference for ancient forests in one or both of the studied forest types. This is a collection of very different species. There are some true forest species (predominantly occurring in closed forests – Schmidt et al. 2011), with short-distance dispersal abilities, like Convallaria majalis, Maianthemum bifolium, and Trientalis europaea, which prefer shade or half-shade as well as fresh, poor acidic soils. They indicate ancient forests in deciduous habitats as well (Heinken 1998; Bossuyt et al. 1999; Dzwonko 2001a; Orczewska, 2010). However, a considerable part of the AFIs also constitute species characteristic of open habitat communities, for example Calamagrostis arundinacea, Calluna vulgaris and Molinia caerulea. Similarly, in the study by Graae and Sunde (2000), some species that grow outside forests in long-established, extensively managed habitats, were better represented in the old forests than the new. This may be explained by the edaphic conditions. In nutrient-poor, acidic forest soils the organic layer is of great functional importance. This soil horizon, weakly developed or non-existent on arable land, is mainly responsible for the nutrient supply of forest vegetation (Leuschner and Rode 1999). Furthermore, microbial communities in soils change with the type of land-use (von Oheimb et al. 2008) – C. vulgaris and M. caerulea have symbiotic relationships with fungi, which is why substantial transformation of the soil environment (caused by agro-technical measures) might be especially destructive for them (Smith and Read 1997). Nonetheless, the persistent and numerous seeds of C. vulgaris (Bossuyt and Hermy 2001) probably enable it to survive adverse periods in small unmanaged areas, and therefore it is quite frequent in old recent forests as well.
Among species with a preference for ancient forests are certain mosses, such as Hylocomium splendens, Pohlia nutans and Sciuro-hypnum oedipodium. These are a very important part of pine and mixed oak-pine forests’ understorey, which is why they should be included as ancient forests indicators in these acidic habitats. Previously, bryophytes were not taken into account as ancient forest indicators. This issue has been taken up recently by Mölder et al. (2015), who emphasized that woodland bryophytes are very sensitive to varying environmental conditions or changes in land management, and compiled a list of ancient woodland indicator bryophytes based on datasets from northern Germany. While our results are not in line with their list of AFIs, the studies cannot be directly compared as we took into account only species related to soil substrate. Most of these grow predominantly in forest, as well as in non-forest areas. Our AFIs, H. splendens and P. nutans, were classified as indifferent or recent forest species in the German study (S.-h. oedipodium was not noticed or not distinguished from similar species in the area). These discrepancies could result from differences in habitat conditions or forest management intensity (e.g. thinning, grazing pressure) – Brunet et al. (1996). In our study, P. nutans was frequently observed in more open stands, on bare soil remaining after disturbance.
Several species that clearly differentiate two studied forest types – Maianthemum bifolium, Polygonatum odoratum and Pteridium aquilinum – have proved to be ancient forest indicators in mixed oak-pine forests, but are rarely observed in pine forests. Their ecological characteristics do not differ much from AFIs in pine forests but they do seem to be sensitive to soil conditions, especially the thickness of the litter layer. A thick layer of slowly decomposing pine litter hampers both recruitment of the large-seed species M. bifolium and P. odoratum (Dzwonko and Gawroński 1994), and of P. aquilinum, with large amounts of small spores that require bare soil for germination (Convay 1957). The litter of mixed oak and pine species decomposes much faster than the pine litter (Cornelissen 1996; Dzwonko 2001b). Moreover, wild boars that often forage in mixed oak-pine forests for acorns, strongly contribute to soil turnover (author’s own observations).
The studied forest types also differ in the recovery of certain species, e.g. Calluna vulgaris and Vaccinium vitis-idaea. These proved to be AFIs in pine forests, but in mixed oak-pine forests were most frequent in the oldest recent stands. This can be explained by the more favourable site conditions – litter and humus type, nutrient availability, microbial activity etc. (Verheyen et al. 2003a; Wulf and Heinken 2008; Orczewska and Fernes 2011), as well as the more efficient soil regeneration processes in richer habitats (Verheyen and Hermy 2001; Wulf 2003; De Keersmaeker et al. 2004).
4.2 Differences from existing AFI lists
A considerable part of the ancient forest indicators according to Hermy et al. (1999) and Dzwonko and Loster (2001) show a preference for ancient stands in our study as well (e.g. C. majalis, M. bifolium and P. aquilinum). Their affinity to ancient forests in pine or mixed oak-pine forest communities was also confirmed by Góras and Orczewska (2007), Orczewska (2007) and Wulf and Heinken (2008). On the other hand, when compared with the aforementioned lists, our results point to different species – for example, Dryopteris carthusiana (Vill.) H.P. Fuchs, Melampyrum pratense L., Mycelis muralis (L.) Dumort., Oxalis acetosella L., Vaccinium myrtillus L. – as occurring as frequently in ancient as in recent forests or with insignificant differences. Differences in these species’ occurrence can be explained by different environmental aspects such as canopy cover, soil type, landscape structure etc. (Graae 2000; Vellend 2003; De Frenne et al. 2011). For instance, Dzwonko and Loster (1990) affirmed that forest communities developing under extreme conditions show less vegetation divergence than those growing under less stringent circumstances. They found that young and mature forests on sandy soils are more similar to each other than young and mature forests on calcareous soils. Moreover, in our study, most of the sampling plots are located within large forest complexes; recent stands are rarely completely isolated in the landscape. This can accelerate the colonization of some species which could migrate from adjacent older forests or other habitats (e.g. clumps of trees on balks), where they survived adverse periods. A similar situation was observed by Graae et al. (2003). Their study demonstrated smaller differences in vegetation in relation to former land-use within the major forest complexes than in isolated new forests. This concerned species such as O. acetosella and M. muralis.
Additionally, most of the species listed above are anemochores or zoochores that can be dispersed over longer distances. For instance, D. carthusiana has very small wind-dispersed spores, which probably explains their ability to colonize isolated stands (Brunet 2007) or even former arable land (Wulf 2004). Some species are also thought to have had a different phytosociological or ecological behaviour in the past. For example, M. pratense was described by Behlen (1833) as being typical of grasslands. Furthermore, we observed significant differences not in the frequency, but in the abundance of some forest plant species. The frequency of V. myrtillus was found to be unaffected by forest persistence, despite reaching much higher cover in ancient forests (see Matuszkiewicz et al. 2013b). The same was observed by Góras and Orczewska (2007), Orczewska and Fernes (2011).
There is also a distinct group of typical forest species which show an affinity for recent forests; the most surprising are declining species from the Ericaceae family (Chimaphila umbelatta (L.) W.P.C. Barton, Pyrola chlorantha Sw.), typical of the Vaccinio-Piceetea class. Their low frequency in ancient forests can be explained by an inability to compete with dwarf-shrub species, such as C. vulgaris or V. myrtillus, which are more abundant in these communities than in recent forests (Matuszkiewicz et al. 2013a).
4.3 Ancient pine forest indicators’ traits
Dispersal-related traits significantly distinguish AFIs from other species found in nutrient-poor forest habitats, and have been linked to poor colonizing ability in many other studies as well (Verheyen et al. 2003b; Kimberley et al. 2013; Kelemen et al. 2014). Most AFIs are short-distance dispersing species with small numbers of heavy seeds; autochores (e.g. T. europaea) or myrmecochores (e.g. Luzula pilosa) are significantly more frequent in this group. Their presence in recent forests can be related to unusual dispersal events, such as vertebrate (including human) dispersal (Hermy and Verheyen 2007). Long-distance dispersal species (anemochores and endozoochores) which are confined to ancient forest have either specific recruitment requirements (e.g. P. aquilinum – Hermy et al. 1999), or may depend on limited animal forage range (Schaumann and Heinken 2002; Graae et al. 2003; Atlegrim 2005). Other traits, such as life span, growth form, height and even seed longevity turn out to be useless in contrast to other studies (Verheyen et al. 2003b; Kimberley et al. 2013; Kelemen et al. 2014). We expected, for instance, that perennial herb species would dominate in ancient forest indicators while annuals and graminoids were more frequent in recent forests. But in the studied mature (at least 60-year-old) recent stands, we observed fewer species remaining after early phases of post-agricultural succession, or that had entered temporarily after disturbance (e.g. annuals). Furthermore, most ancient forest species are thought to produce short-living seeds, and their ecological restoration cannot rely on the seed bank, but seed banks of coniferous forests contain more light-demanding, small seeded and heathland species (e.g. C. vulgaris) which are known to remain viable for long periods in the soil, though their recruitment requirements often remain unfulfilled (Bossuyt and Hermy 2001). However, there are also true forest species having a permanent seed bank, such as L. pilosa. This may explain why this species has an affinity for recent forests in some studies (Orczewska 2007; Wulf and Heinken 2008; Orczewska and Fernes 2011). SLA does not differ much between AFIs and non-AFIs. High SLA has been associated with both shade tolerance and resource-rich environments (Pérez-Herguindeguy et al. 2013). However, in nutrient-poor, half-shaded forest habitats, its value is somewhat low in both species groups, although it does reflect habitat differences between the studied forest types.
5 Conclusions
There are several herb and moss species that can be associated with ancient pine and/or mixed oak-pine forests. However, there are no herbaceous plant or bryophyte species that grow exclusively on ancient forest sites. To identify an ancient forest site with a high degree of accuracy, not single, but several ancient forest indicators should be detected. The necessary number of species ranges in the literature from 2 to 27 (Schmidt et al. 2014).
Although the low colonizing capacity cannot be attributed to a single cause, dispersal-related traits seem to be the most important in the profile characteristics of ancient forest indicators in pine and mixed oak-pine forests. Therefore, forest habitat availability in the landscape has a crucial role in recent forest regeneration. We should stress the need to avoid ancient forest clearance and fragmentation of woodland; afforestation should be located in the vicinity of ancient stands. Although protection of ancient forest species requires the maintenance of their habitats, this should not exclude forestry. Management practices such as sustainable canopy thinning or use as wood-pasture were normal in earlier times, and did not break the continuity of woodland at the site. Most typical forest plants are well adapted to – and partly depend on – the occurrence of canopy gaps and soil disturbance (Wulf 1997). For example, periodic soil turnover may even be necessary, providing patches of bare soil for some plants’ establishment (e.g. P. aquilinum – Brunet et al. 1996). Moreover, recent forests have turned out to support several rare plant species. Therefore, to maintain phytodiversity at the landscape level, a mixture of ancient and recent forests, both managed and strictly protected, is needed.
Acknowledgements
This research was supported by the Polish Ministry of Science and Higher Education (Research Project No. NN 305 0800835). We thank two anonymous reviewers and editors for their constructive comments.
References
Atlegrim O. (2005). Indirect effects of ant predation (Hymenoptera: Formicidae) on bilberry Vaccinium myrtillus. European Journal of Entomology 102(2): 175–180. http://dx.doi.org/10.14411/eje.2005.028.
Behlen S. (ed.) (1833). Dr. Joh. Matth. Bechstein’s Forstbotanik. 2. Teil. Forstkräuterkunde oder Naturgeschichte der deutschen Forstkräuter. Henning, Erfurt.
Bekker R.M., Schaminee J.H.J., Bakker J.P., Thompson K. (1998a). Seed bank characteristics of Dutch plant communities. Acta Botanica Neerlandica 47: 15–26.
Bekker R.M., Bakker J.P., Grandin U., Kalamees R., Milberg P., Poschlod P., Thompson K., Willems J.H. (1998b). Seed size, shape and vertical distribution in the soil: indicators of seed longevity. Functional Ecology 12(5): 834–843. http://dx.doi.org/10.1046/j.1365-2435.1998.00252.x.
Bellemare J., Motzkin G., Foster D.R. (2002). Legacies of the agricultural past in the forested present: an assessment of historical land-use effects on rich mesic forests. Journal of Biogeography 29: 1401–1420. http://dx.doi.org/10.1046/j.1365-2699.2002.00762.x.
Bossuyt B., Deckers J., Hermy M. (1999). A field methodology for assessing man-made disturbance in forest soils developed in loess. Soil Use and Management 15(1): 14–20. http://dx.doi.org/10.1111/j.1475-2743.1999.tb00056.x.
Bossuyt B., Hermy M. (2001). Influence of land use history on seed banks in European temperate forest ecosystems: a review. Ecography 24: 225–238. http://dx.doi.org/10.1034/j.1600-0587.2001.240213.x.
Braun-Blanquet J. (1964). Pflanzensoziologie, Grundzüge der Vegetationskunde. Auflage:3. Springer, Wien-New York.
Brunet J. (2007). Plant colonization in heterogeneous landscapes: an 80-year perspective on restoration of broadleaved forest vegetation. Journal of Applied Ecology 44: 563–572. http://dx.doi.org/10.1111/j.1365-2664.2007.01297.x.
Brunet J., von Oheimb G. (1998). Migration of vascular plants to secondary woodlands in southern Sweden. Journal of Ecology 86: 429–438. http://dx.doi.org/10.1046/j.1365-2745.1998.00269.x.
Brunet J., Falkengren-Grerup U., Tyler G. (1996). Herb layer vegetation of south Swedish beech and oak forests – effects of management and soil acidity during one decade. Forest Ecology and Management 88: 259–272. http://dx.doi.org/10.1016/S0378-1127(96)03845-5.
Convay E. (1957). Spore production in bracken. Journal of Ecology 45(1): 273–284. http://dx.doi.org/10.2307/2257089.
Cornelissen J.H.C. (1996). An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. Journal of Ecology 84: 573–582. http://dx.doi.org/10.2307/2261479.
De Frenne P., Beaten L., Graae B.J., Brunet J., Wulf M., Orczewska A., Kolb A., Jansen I., Jamoneau A., Jacquemyn H., Hermy M., Diekmann M., De Schrijver A., De Sanctis M., Decocq G., Cousins S.A.O., Verheyen K. (2011). Interregional variation in the floristic recovery of post-agricultural forests. Journal of Ecology 99(2): 600–609. http://dx.doi.org/10.1111/j.1365-2745.2010.01768.x.
De Keersmaeker L., Martens L., Verheyen K., Hermy M., De Schrijver A., Lust N. (2004). Impact of soil fertility and insolation on diversity of herbaceous woodland species colonizing afforestations in Muizen forest (Belgium). Forest Ecology and Management 188: 291–304. http://dx.doi.org/10.1016/j.foreco.2003.07.025.
Distribution map of Scots pine (Pinus sylvestris) (2009). EUFORGEN – the European Forest Genetic Resources Programme. http://www.euforgen.org.
Düll R., Kutzelnigg H. (1986). Neues botanisch – okologishes Excursionstaschenbuch. IDH, Rheurdt.
Dzwonko Z. (2001a). Migration of vascular plant species to a recent wood adjoining ancient woodland. Acta Societatis Botanicorum Poloniae 70(1): 71–77. http://dx.doi.org/10.5586/asbp.2001.010.
Dzwonko Z. (2001b). Effect of proximity to ancient deciduous woodland on restoration of the field layer vegetation in a pine plantation. Ecography 24: 198–204. http://dx.doi.org/10.1034/j.1600-0587.2001.240210.x.
Dzwonko Z. (2007). Przewodnik do badań fitosocjologicznych. [A guide for phytosociological studies]. Institute of Botany, Jagiellonian University, Sorus, Poznań, Kraków.
Dzwonko Z., Gawroński S. (1994). The role of woodland fragments, soil types, and dominant species in secondary succession on the western Carpathian foothills. Vegetatio 111(2): 149–160. http://dx.doi.org/10.1007/BF00040334.
Dzwonko Z., Loster S. (1988). Species richness of small woodlands on the western Carpathian foothills. Vegetatio 76: 15–27.
Dzwonko Z., Loster S. (1990). Vegetation differentiation and secondary succession on a limestone hill in southern Poland. Journal of Vegetation Science 1: 615–622. http://dx.doi.org/10.2307/3235567.
Dzwonko Z., Loster S. (2001). Wskaźnikowe gatunki roślin starych lasów i ich znaczenie dla ochrony przyrody i kartografii roślinności. [Ancient woodland plant species indicators and their importance for nature conservation and vegetation mapping]. Prace Geograficzne 178: 119–132.
Eriksson O. (1995). Seedling recruitment in deciduous forest herbs: the effects of litter, soil chemistry and seed bank. Flora 190: 65–70.
Faliński J.B. (1986). Vegetation dynamics in temperate lowland primeval forests. Geobotany 8: 1–537. http://dx.doi.org/10.1007/978-94-009-4806-8.
Falkengren-Grerup U., Ten Brink D.J., Brunet J. (2006). Land use effects on soil N, P, C and pH persist over 40–80 years of forest growth on agricultural soils. Forest Ecology and Management 225(1–3): 74–81. http://dx.doi.org/10.1016/j.foreco.2005.12.027.
Flinn K.M., Vellend M. (2005). Recovery of forest plant communities in post-agricultural landscapes. Frontiers in Ecology and the Environment 3: 243–250. http://dx.doi.org/10.1890/1540-9295(2005)003%5B0243:ROFPCI%5D2.0.CO;2.
Forest Europe, UNECE, FAO (2011). State of Europe’s forests 2011. Status and trends in sustainable forest management in Europe. Forest Europe, UNECE and FAO, Oslo.
Forestry. Statistical Yearbook of the Republic of Poland (2013). Central Statistical Office of Poland, Warsaw.
Goldberg E., Kirby K., Hall J., Latham J. (2007). The ancient woodland concept as a practical conservation tool in Great Britain. Journal for Nature Conservation 15: 109–119. http://dx.doi.org/10.1016/j.jnc.2007.04.001.
Góras P., Orczewska A. (2007). Zróżnicowanie runa w lasach sosnowych posadzonych na gruntach porolnych i w starych lasach sosnowych na siedlisku boru mieszanego świeżego. [Differentiation of the herbaceous layer of the post-arable and ancient pine forests developing on a habitat of recent mixed pine forests]. Przegląd Przyrodniczy 18(1–2): 227–241.
Graae B.J. (2000). The effect of landscape fragmentation on forest floor species in two regions of Denmark. Journal of Vegetation Science 11(6): 881–892. http://dx.doi.org/10.2307/3236558.
Graae B.J., Heskjaer V.S. (1997). A comparison of understorey vegetation between untouched and managed deciduous forest in Denmark. Forest Ecology and Management 96: 111–123. http://dx.doi.org/10.1016/S0378-1127(97)00046-7.
Graae B.J., Sunde P.B. (2000). The impact of forest continuity and management on forest floor vegetation evaluated by species traits. Ecography 23(6): 720–731. http://dx.doi.org/10.1111/j.1600-0587.2000.tb00315.x.
Graae B.J., Sunde P.B., Fritzbøger B. (2003). Vegetation and soil differences in ancient opposed to new forests. Forest Ecology and Management 177: 179–190. http://dx.doi.org/10.1016/S0378-1127(02)00438-3.
Heinken T. (1998). Zum Einfluss des Alters von Waldstandorten auf die Vegetation in bodensauren Laubwäldern des Niedersächsischen Tieflandes. Archiv für Naturschutz und Landschaftsforschung 37: 201–232.
Heinken T. (2008). Vaccinio-Piceetea (H7) – Beerstrauch-Nadelwälder, Teil 1: Dicrano-Pinion – Sand- und Silikat-Kiefernwälder. In: Dierschke H. (ed.). Synopsis der Pflanzengesellschaften Deutschlands. Die Arbeitsgemeinschaft, Göttingen. 88 p.
Hermy M., Stieperaere H. (1981). An indirect gradient analysis of the ecological relationships between ancient and recent riverine woodlands to the south of Brughes (Flanders, Belgium). Vegetatio 44: 43–49. http://dx.doi.org/10.1007/BF00119802.
Hermy M., Verheyen K. (2007). Legacies of the past in the present-day forest biodiversity: a review of past land-use effects on forest plant species composition and diversity. Ecological Research 22: 361–371. http://dx.doi.org/10.1007/s11284-007-0354-3.
Hermy M., Honnay O., Firbank L., Grashof-Bokdam C., Lawesson J.E. (1999). An ecological comparison between ancient and other forest plant species of Europe, and the implications for forest conservation. Biological Conservation 91: 9–22. http://dx.doi.org/10.1016/S0006-3207(99)00045-2.
Honnay O., Degroote B., Hermy M. (1998). Ancient-forest plant species in western Belgium: a species list and possible ecological mechanisms. Belgian Journal of Botany 130: 139–154.
Jankowska-Błaszczuk M., Grubb P.J. (1997). Soil seed banks in primary and secondary deciduous forest in Białowieża, Poland. Seed Science Research 7: 281–292. http://dx.doi.org/10.1017/S0960258500003639.
Kelemen K., Kriván A., Standovár T. (2014). Effects of land-use history and current management on ancient woodland herbs in Western Hungary. Journal of Vegetation Science 25: 172–183. http://dx.doi.org/10.1111/jvs.12046.
Kimberley A., Blackburn G.A., Whyatt J.D., Kirby K., Smart S.M. (2013). Identifying the trait syndromes of conservation indicator species: how distinct are British ancient woodland indicator plants from other woodland species? Applied Vegetation Science 16: 667–675. http://dx.doi.org/10.1111/avsc.12047.
Kleyer M., Bekker R.M., Knevel I.C., Bakker J.P., Thompson K., Sonnenschein M., Poschlod P., Van Groenendael J.M., Klimeš L., Klimešová J., Klotz S., Rusch G.M., Hermy M., Adriaens D., Boedeltje G., Bossuyt B., Dannemann A., Endels P., Götzenberger L., Hodgson J.G., Jackel A.-K., Kühn I., Kunzmann D., Ozinga W.A., Römermann C., Stadler M., Schlegelmilch J., Steendam H.J., Tackenberg O., Wilmann B., Cornelissen J.H.C., Eriksson O., Garnier E., Peco B. (2008). The LEDA traitbase: a database of life-history traits of the NW European flora. Journal of Ecology 96(6): 1266–1274. http://dx.doi.org/10.1111/j.1365-2745.2008.01430.x.
Kuiters A.T., Denneman C.A.J. (1987). Water-soluble phenolic substances in soil under several coniferous and deciduous tree species. Soil Biology and Biochemistry 19: 765–769. http://dx.doi.org/10.1016/0038-0717(87)90061-7.
Leuschner C., Rode M.W. (1999). The role of plant resources in forest succession: changes in radiation, water and nutrient fluxes, and plant productivity over a 300-year-long chronosequence in NW-Germany. Perspectives in Plant Ecology, Evolution and Systematics 2: 103–147. http://dx.doi.org/10.1078/1433-8319-00067.
Lindacher R. (ed.) (1995). Phanart Datenbank der Gefässpflanzen Mitteleuropas, Erklärung der Kennzahlen, Aufbau und Inhalt. [Phanart, database of Central European vascular plants, explanation of codes, structure and contents]. Veröffentlichungen des Geobotanischen Institut der ETH Stiftung Rübel, Zürich.
Marcos J.A., Marcos E., Taboada A., Tárrega R. (2007). Comparison of community structure and soil characteristics in different aged Pinus sylvestris plantations and a natural pine forest. Forest Ecology and Management 247: 35–42. http://dx.doi.org/10.1016/j.foreco.2007.04.022.
Matuszkiewicz J.M., Kowalska A., Solon J., Degórski M., Kozłowska A., Roo-Zielińska E., Zawiska I., Wolski J. (2013a). Long-term evolution models of post-agricultural forests. Prace Geograficzne 240.
Matuszkiewicz J.M., Kowalska A., Kozłowska A., Roo-Zielińska E., Solon J. (2013b). Differences in plant-species composition, richness and community structure in ancient and post-agricultural pine forests in central Poland. Forest Ecology and Management 310: 567–576. http://dx.doi.org/10.1016/j.foreco.2013.08.060.
Matuszkiewicz J.M., Wolski J., Kowalska A. (2013c). A map of sequences of ’forest/non-forest states’ over the last 200 years in the borderland between Poland’s Masuria and Kurpie regions. Geographia Polonica 86(4): 393–402. http://dx.doi.org/10.7163/GPol.2013.31.
Matuszkiewicz W. (2001). Przewodnik do oznaczania zbiorowisk roślinnych Polski. [A guide for the identification of Polish plant communities]. Wydawnictwo Naukowe PWN, Warszawa.
McCune B., Mefford M.J. (2011). PC-ORD. Multivariate analysis of ecological data. Version 6. MjM Software, Gleneden Beach, Oregon, USA.
Mölder A., Schmidt M., Engel F., Schönfelder E., Schulz F. (2015). Bryophytes as indicators of ancient woodlands in Schleswig-Holstein (Northern Germany). Ecological Indicators 54: 12–30. http://dx.doi.org/10.1016/j.ecolind.2015.01.044.
Müller-Schneider P. (1986). Verbreitungsbiologie der Blütenpflanzen. Veröff. Geobot. Inst. ETH, Zürich, Stiftung Rübel 85. 263 p.
Nordén B., Appelqvist T. (2001). Conceptual problems of ecological continuity and its bioindicators. Biodiversity and Conservation 10: 779–791. http://dx.doi.org/10.1023/A:1016675103935.
Orczewska A. (2007). Znaczenie starych lasów w procesie renaturalizacji runa leśnego w lasach wtórnych pochodzenia porolnego. [Importance of ancient forests in understorey recovery in recent post-agricultural forests]. Studia i Materiały Centrum Edukacji Przyrodniczo-Leśnej 9(2–3): 356–369.
Orczewska A. (2010). Colonization capacity of herb woodland species in fertile, recent alder woods adjacent to ancient forest sites. Polish Journal of Ecology 58: 297–310.
Orczewska A., Fernes M. (2011). Migration of herb layer species into the poorest post-agricultural pine woods adjacent to ancient pine forests. Polish Journal of Ecology 59: 113–123.
Pérez-Harguindeguy N., Diaz S., Garnier E., Lavorel S., Poorter H., Jaureguiberry P., Bret-Harte M.S., Cornwell W.K., Craine J.M., Gurvich D.E., Urcelay C., Veneklaas E.J., Reich P.B., Poorter L., Wright I.J., Ray P., Enrico L., Pausas J.G., de Vos A.C., Buchmann N., Funes G., Quétier F., Hodgson J.G., Thompson K., Morgan H.D., ter Steege H., van der Heijden M.G.A., Sack L., Blonder B., Poschlod P., Vaieretti M.V., Conti G., Staver A.C., Aquino S., Cornelissen J.H.C. (2013). New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61(3): 167–234. http://dx.doi.org/10.1071/BT12225.
Peterken G.F. (1974). A method for assessing woodland flora for conservation using indicator species. Biological Conservation 6: 239–245. http://dx.doi.org/10.1016/0006-3207(74)90001-9.
Peterken G.F. (1977). Habitat conservation priorities in British and European woodlands. Biological Conservation 11: 223–236. http://dx.doi.org/10.1016/0006-3207(77)90006-4.
Petersen P.M. (1994). Flora, vegetation, and soil in broadleaved ancient and planted woodland, and scrub on Røsnœs, Denmark. Nordic Journal of Botany 14: 693–709. http://dx.doi.org/10.1111/j.1756-1051.1994.tb01086.x.
Rackham O. (1980). Ancient woodland its history, vegetation and uses in England. Arnold, London.
Reinecke J., Klemm G., Heinken T. (2014). Vegetation change and homogenization of species composition in temperate nutrient deficient Scots pine forests after 45 yr. Journal of Vegetation Science 25(1): 113–121. http://dx.doi.org/10.1111/jvs.12069.
Rolstad J., Gjerde I., Gundersen V.S., Saetersal M. (2002). Use of indicator species to assess forest continuity: a critique. Conservation Biology 16: 253–257. http://dx.doi.org/10.1046/j.1523-1739.2002.00552.x.
Rose F. (1999). Indicators of ancient woodland – the use of vascular plants in evaluating ancient woods for nature conservation. British Wildlife 10: 241–251.
Schaumann F., Heinken T. (2002). Endozoochorous seed dispersal by martens (Martes foina, M. martes) in two woodland habitats. Flora 197: 370–378. http://dx.doi.org/10.1078/0367-2530-00053.
Schmidt M., Kriebitzsch W.-U., Ewald J. (2011). Waldartenlisten der Farn-und Blütenpflanzen, Moose und Flechten Deutschlands. BfN-Skripten 299: 1–111.
Schmidt M., Mölder A., Schönfelder E., Engel F., Schmiedel I., Culmsee H. (2014). Determining ancient woodland indicator plants for practical use: a new approach developed in northwest Germany. Forest Ecology and Management 330: 228–239. http://dx.doi.org/10.1016/j.foreco.2014.06.043.
Smith S., Read D. (1997). Mycorrhizal symbiosis. 2nd ed. Cambridge University Press.
Summers R.W., Mavor R.A., MacLennan A.M., Rebecca G.W. (1999). The structure of ancient native pinewoods and other woodlands in the Highlands of Scotland. Forest Ecology and Management 119: 231–245. http://dx.doi.org/10.1016/S0378-1127(98)00515-5.
Thompson K., Bakker J.P., Bekker R.M., Hodgson J.G. (1998). Ecological correlates of seed persistence in soil in the north-west European flora. Journal of Ecology 86: 163–169. http://dx.doi.org/10.1046/j.1365-2745.1998.00240.x.
Tichý L., Chytrý M. (2006). Statistical determination of diagnostic species for site groups of unequal size. Journal of Vegetation Science 17: 809–818. http://dx.doi.org/10.1111/j.1654-1103.2006.tb02504.x.
Vellend M. (2003). Habitat loss inhibits recovery of plant diversity as forests regrow. Ecology 84: 1158–1164. http://dx.doi.org/10.1890/0012-9658(2003)084%5B1158:HLIROP%5D2.0.CO;2.
Verheyen K., Hermy M. (2001). The relative importance of dispersal limitation of vascular plants in secondary forest succession in Muizen Forest, Belgium. Journal of Ecology 89: 829–840. http://dx.doi.org/10.1046/j.0022-0477.2001.00596.x.
Verheyen K., Guntenspergen G.R., Biesbrouck B., Hermy M. (2003a). An integrated analysis of the effects of past land use on forest herb colonization at the landscape scale. Journal of Ecology 91: 731–742. http://dx.doi.org/10.1046/j.1365-2745.2003.00807.x.
Verheyen K., Honnay O., Motzkin G., Hermy M., Foster D.R. (2003b). Response of forest plant species to land-use change: a life-history trait-based approach. Journal of Ecology 91: 563–577. http://dx.doi.org/10.1046/j.1365-2745.2003.00789.x.
von Oheimb G., Härdtle W., Naumann P.S., Westphal C., Assmann T., Meyer H. (2008). Long-term effects of historical heathland farming on soil properties of forest ecosystems. Forest Ecology and Management 255(5–6): 1984–1993. http://dx.doi.org/10.1016/j.foreco.2007.12.021.
Witkowska-Żuk L. (2008). Atlas roślinności lasów. [Atlas of forest vegetation]. MULTICO Oficyna Wydawnicza, Warszawa.
Wulf M. (1997). Plant species as indicators of ancient woodland in north-western Germany. Journal of Vegetation Science 8: 635–642. http://dx.doi.org/10.2307/3237367.
Wulf M. (2003). Preference of plant species for woodlands with different habitat continuities. Flora 198: 444–460. http://dx.doi.org/10.1078/0367-2530-00118.
Wulf M. (2004). Plant species richness of afforestations with different former use and habitat continuity. Forest Ecology and Management 195: 191–204. http://dx.doi.org/10.1016/j.foreco.2004.02.046.
Wulf M., Heinken T. (2008). Colonization of recent coniferous versus deciduous forest stands by vascular plants at the local scale. Applied Vegetation Science 11: 307–316. http://dx.doi.org/10.3170/2008-7-18432.
Total of 86 references.

