Bilberry ramet dimensions in relation to stand age in oligotrophic conditions in Latvia
Robalte L., Jansone D., Elferts D., Matisons R., Jansons Ā. (2018). Bilberry ramet dimensions in relation to stand age in oligotrophic conditions in Latvia. Silva Fennica vol. 52 no. 1 article id 9899. https://doi.org/10.14214/sf.9899
Highlights
- Bilberry ramet dimensions (age, diameter, height) and their structural diversity, as well as cover, increased with stand age
- Active rejuvenation of ramets was observed in younger stands
- The oldest bilberry ramets (>10 years of age) occurred in stands older than 70 years.
Abstract
Dwarf shrub layer is an important component of boreal and hemiboreal forest ecosystems that has received little attention, particularly regarding its structural diversity, which, however, could serve as an additional proxy for habitat quality. Dimensions of bilberry (Vaccinium myrtillus L.) ramets were assessed in two sites in Latvia covered by dry oligotrophic Scots pine (Pinus sylvestris L.) stands 10–230 years of age. In total, 20 sampling plots (10×10 m) with 156 subplots (1×1 m) were sampled and 630 bilberry ramets analysed. The dimensions of ramets (age, diameter, and height) and cover of bilberry increased with stand age. The age of the studied ramets ranged 2–13 years; 5–6 years-old ramets were most frequent in all stands. The skewness of the distribution of the ramet dimensions shifted with stand age, leaning towards the higher values. Lower structural diversity of ramets was observed in stands 50–100 years of age. The highest diversity of ramet age structure occurred in stands younger than 150 years, whereas the oldest and largest ramets mostly occurred in the older stands (>150 years). Considering structural diversity of ramets, recovery of bilberry after stand-replacing disturbance (e.g. clearcut) was a continuous process, similarly to that observed in tree layer.
Keywords
structural diversity;
Vaccinium myrtillus;
dwarf shrubs;
projective cover;
hemiboreal forests
-
Robalte,
Latvian State Forest Research Institute “Silava”, 111 Rigas Str., LV 2169, Salaspils, Latvia
E-mail
robalte.l@gmail.com

- Jansone, Latvian State Forest Research Institute “Silava”, 111 Rigas Str., LV 2169, Salaspils, Latvia; University of Latvia, Faculty of Biology, Jelgavas Str. 1, LV 1004, Riga, Latvia E-mail diana.jansone13@gmail.com
- Elferts, Latvian State Forest Research Institute “Silava”, 111 Rigas Str., LV 2169, Salaspils, Latvia; University of Latvia, Faculty of Biology, Jelgavas Str. 1, LV 1004, Riga, Latvia E-mail didzis.elferts@lu.lv
- Matisons, Latvian State Forest Research Institute “Silava”, 111 Rigas Str., LV 2169, Salaspils, Latvia E-mail roberts.matisons@silava.lv
- Jansons, Latvian State Forest Research Institute “Silava”, 111 Rigas Str., LV 2169, Salaspils, Latvia E-mail aris.jansons@silava.lv
Received 1 November 2017 Accepted 1 February 2018 Published 1 February 2018
Views 60690
Available at https://doi.org/10.14214/sf.9899 | Download PDF

1 Introduction
Comprehensive information on ecological processes, which is commonly based on tree stand and understorey vegetation (Nilsson and Wardle 2005), is required for sustainable forest management. In oligotrophic boreal forests, dwarf shrubs, e.g. bilberry (Vaccinium myrtillus L.) produce reasonable amount of biomass (Jäderlund et al. 1996; Nestby et al. 2011), positively affects soil properties (Wallstedt et al. 2005), and plays considerable part in trophic chains (Nilsson and Wardle 2005). In stands on mineral soil, genetic and morphologic diversity of bilberry is high, aiding to resilience of the ecosystem to ecological changes (Albert et al. 2004; Nilsson and Wardle 2005). Nevertheless, bilberry is sensitive to light deficit under canopy (Miina et al. 2009), although excessive solar radiation in open conditions, e.g. after clearcut, can be devastating (Ritchie 1956; Tolvanen 1995). After clearcut or stand-replacing disturbances, bilberry recovers successfully, yet the dimensions of the ramets are affected and complete recovery is time consuming (Flower-Ellis 1971; Lõhmus and Remm 2017). Still, a few studies have dealt with bilberry ramet structure and its interaction with environmental factors (e.g. Flower-Ellis 1971; Albert et al. 2004; Rixen et al. 2004).
Considering formation of annual rings, it is possible to assess age structure of bilberry ramets (Flower-Ellis 1971; Schweingruber and Poschlod 2005; Nielsen et al. 2007), which similarly to diameter and height, might serve as a proxy for habitat quality (Ritchie 1956; Flower-Ellis 1971; Schweingruber and Poschlod 2005), hence as an additional census of ecosystem quality (Kuuluvainen and Aakala 2011). In addition, bilberry ramets at the age of 3–5 years have the highest intensity of regeneration and growth (Tolvanen 1995). Hence information about ramet age can also be related to gross productivity of stands. This study aimed to assess the effect of stand age on age, diameter, and height of bilberry ramets in oligotrophic pine stands in Latvia. We assumed that mean age, height, and diameter of bilberry ramets, as well as their structure diversity increased with stand age.
2 Materials and methods
2.1 Study site, sampling and sample preparation
Two lowland sites with a flat topography (Melnsils 57°39´N, 22°34´E; Vangaži 56°58´N, 24°29´E), covered by oligotrophic Scots pine stands (Vacciniosa) 10–230 years of age, were studied. At the Vangaži site, stands were managed (≤20 years since thinning), yet no management occurred at the Melnsils site since 1980s. In these sites, climate was mild, the mean monthly temperature (±standard deviation) ranged from 17.4 ± 1.6 °C in July to –3.8 ± 4 °C in February. Annual sum of precipitation was 693 ± 30 mm (Harris et al. 2014).
Twelve sampling plots (10×10 m) were established in Melnsils and eight in Vangaži sites (one or two per stand). Sampling plots were placed in forest patches, where bilberry cover was >50%. In each sampling plot, 6–8 randomly distributed subplots (1×1 m) were established (156 in total). Considering location of sampling plot, we assumed that mostly each subplot was occupied by a different genet (Albert et al. 2004). In each subplot, cover (%) of the ground understorey vegetation (by species) was recorded, and four to five bilberry ramets (with rhizome; touching each corner and the centre of the subplots) were collected if present at those points (630 in total).
In laboratory, height (length) of the aboveground part (from root collar to the top apical bud) of ramets was measured (±0.1 cm). Stem diameter was measured in two perpendicular directions at root collar (i.e. above the rhizome; ±0.1 mm), and a relevant stem disc was taken. From the stem disks, thin sections (10–60 μm) were cut with a hand microtome, dyed in safranin and astrablue, and sealed in Canada Balm (Schweingruber and Poschlod 2005). Annual rings were counted under a microscope.
2.2 Data analysis
Linear mixed effect models were used to assess influence of stand age (fixed effect) on dimensions (age, diameter and height) of the ramets as well as on cover of bilberry; plots and subplots were used as random effects. To assess the effect of structure of ground cover vegetation on bilberry ramet parameters, cover of other dwarf shrubs, herbaceous plant and moss cover were added to the models as numeric covariates. Log transformation was applied for the cover data, as they were heteroscedastic and asymmetrically distributed.
To assess structural diversity of bilberry, ramet age, diameter, and height was divided into seven equal classes; stands were divided in five age classes: <50; 51–100; 101–150; 151–200; ≥201 years of age. Skewness and Simpson evenness indices were calculated for description of the distributions. Data analysis was conducted using program R 3.4.2. (R Core Team 2017) and package “lme4” (Bates et al. 2013).
3 Results
Overall, the mean cover of bilberry was 27%. The height of ramets ranged 10.7–61.3 cm, with the mean value of 27.0 cm (Fig. 1). Stem diameter of the ramets also had considerable range, as the mean, minimum, and maximum values were 3.0, 0.8, and 7.7 mm, respectively. The largest (above the 3rd quartile) ramets occurred in 101–150 years old stands. The age of ramets at root collar ranged 2–13 years with the mean of 5 years; the oldest (>10 years) ramets mostly occurred in 101–200 years-old stands. The distributions of ramet dimensions were skewed towards the smaller values.
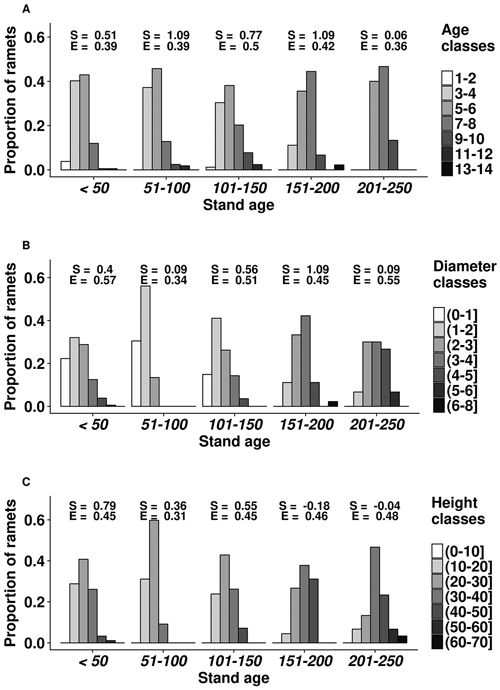
Fig. 1. Age (A), diameter (mm) (B) and height (cm) (C) structure of bilberry ramets in the studied oligotrophic Scots pine (Vacciniosa type) stands. Ordinate axis shows the proportion of the sampled ramets. The skewness (S) and Simpsons evenness (E) of the distributions are shown.
Stand age had a significant (p-values < 0.01) and positive effect on age, diameter and height of the ramets as well as on cover of bilberry in the studied plots. The slope parameters ± standard errors of the linear models for ramet age, diameter, and height were 0.0074 ± 0.0023, 0.0040 ± 0.0012, and 0.0363 ± 0.0113, respectively; while for bilberry cover, the slope parameter was 0.0051 ± 0.0019. Cover of other dwarf shrubs, herbaceous plant and mosses did not show a significant effect on bilberry ramet parameters (p-values ≥ 0.38).
The structural diversity of the studied dimensions of bilberry ramets was influenced by stand age (Fig. 1). Distribution of age, height, and diameter of ramets leaned towards the higher values (positive skewness) with increasing stand age. Ramet structure was more heterogenic (lower evenness index) in 51–100 years old stands. Still, ramet age class of 5–6 years was the most frequent in all stands, except in the oldest ones (201–250 years) (Fig. 1). The number and representation of ramet diameter and height classes increased with the stand age, although it was also high in the younger (<50 years) stands. The effect of stand age on ramet age appeared non-linear, as the highest diversity of ramet age classes was observed in 101–150 years old stands, yet it decreased in older stands.
4 Discussion
The size of the studied ramets was similar to that observed in boreal forests (cf. Flower-Ellis 1971), suggesting comparable growing conditions. Bilberry ramets with diameter >0.8 mm and height >10 cm where found, indicating that such dimension were reached in the first year of their life. Although in harsh environments, age of ramets can reach 100 years (cf. Schweingruber and Poschlod 2005), in this study ramets were ≤13 years old, similarly as observed by Albert et al. (2004) in the nemoral zone, indicating dynamic rejuvenation of bilberry. Such continuous rejuvenation, suggested high gross productivity of bilberry genets (Tolvanen 1995).
Stand age, which is one of the main factors indicating biological diversity of forest ecosystems (Kuuluvainen and Aakala 2011), had a positive effect on size of ramets (Fig. 1), irrespectively of composition of ground cover vegetation, suggesting ongoing recover after stand-replacing disturbances (Flower-Ellis 1971; Lõhmus and Remm 2017). Alternatively, increase in ramet dimensions (Fig. 1) with the maturation of stands might be related to accumulation of nutrients (Mäkipää 1999; Nielsen et al. 2007) and improvement of light conditions due to self-thinning of tree layer, resulting in better growing conditions for bilberry (Janke 1970). Positive reaction of bilberry to low intensity thinning has been observed by Atlegrim and Sjöberg (1996). The cover of bilberry, which also might be used as indicator of site quality (Nilsson and Wardle 2005), increased with age.
The diversity of ramet size increased with stand age, increasing spatial heterogeneity of bilberry cover, hence the number of ecological niches (Haysom and Coulson 1998). Stand-age-related increase in ramet structural diversity might also be related to increasing genetic diversity of bilberry, as new genets might have recruited (Albert et al. 2004). Nevertheless, the shift of the distributions of ramet size to larger values in the older stands (Fig. 1) suggested maturation of bilberry (Jonsdottir, Callaghan 1988). The positive relationships between stand and ramet age, as well as frequency of ramets with the age of 3–5 years, which show the fastest rejuvenation and highest gross productivity (Tolvanen 1995), decreased in the older stands (>201 years), supporting maturation of bilberry. Regarding ramet age, 101–150 years-old stands appeared most suitable for bilberry, as indicated by the highest diversity of ramet age (presence of older individuals) and high frequency of the five-year-old ramets (Fig. 1). The increased diversity of ramet size and decreased ramet age in the younger stands might be related to decreased competition and recovery after the clearcut (Schaal 1978), when productivity of bilberry is high (Dudt and Shure 1994).
5 Conclusions
In the dwarf shrub layer in dry oligotrophic Scots pine stands, dimensions of bilberry ramets showed continuous increase with stand age, indicating continuous maturation of genets. The structural diversity of bilberry ramets, which might be related to number of ecological niches, also tended to increase with stand age, although, ramets of diverse dimensions were observed in the younger stands (<50 years after clearcut). Still, the most diverse ramet age structure was observed in 100–150 years-old stands. The younger ramets, which show the highest productivity, were the most common in <150 years old stands, indicating rejuvenation of bilberry cover.
Acknowledgements
The study was supported by the European Social Fund (ESF) project “Support for Master Studies at the University of Latvia” (No. 2011/0015/1DP/1.1.2.1.1./11/IPIA/VIAA/008) and funded by Joint Stock Company “Latvijas Valsts Meži” (Latvia’s State Forests). We also acknowledge Iluta Dauškane for help during sample preparation.
References
Albert T., Raspé O., Jacquemart A.L. (2004). Clonal diversity and genetic structure in Vaccinium myrtillus populations from different habitats. Belgian Journal of Botany 137(2): 155–162. http://www.jstor.org/stable/20794549.
Atlegrim O., Sjöberg K. (1996). Response of bilberry (Vaccinium myrtillus) to clear-cutting and single-tree selection harvests in uneven-aged boreal Picea abies forests. Forest Ecology and Management 86(1–3): 39–50. https://doi.org/10.1016/S0378-1127(96)03794-2.
Bates D., Maechler M., Bolker B., Walker S. (2013). lme4: linear mixed-effects models using Eigen and s4. R package version 1.0–5. http://CRAN.Rproject.org/package=lme4.
Dudt J.F., Shure D.J. (1994). The influence of light and nutrients on foliar phenols and insect herbivory. Ecology 75(1): 86–98. https://doi.org/10.2307/1939385.
Flower-Ellis J.G.K. (1971). Age structure and dynamics in stands of bilberry (Vaccinium myrtillus L.). Research Notes. Royal College of Forestry, Stockholm. 107 p.
Harris I.P., Jones P.D., Osborn T.J., Lister D.H. (2014). Updated high-resolution grids of monthly climatic observations – the CRU TS3. 10 dataset. International Journal of Climatology 34(3): 623–642. https://doi.org/10.1002/joc.3711.
Haysom K.A., Coulson J.C. (1998). The Lepidoptera fauna associated with Calluna vulgaris: effects of plant architecture on abundance and diversity. Ecological Entomology 23(4): 377–385. https://doi.org/10.1046/j.1365-2311.1998.00152.x.
Jäderlund A., Zackrisson O., Nilsson M.C. (1996). Effects of bilberry (Vaccinium myrtillus L.) litter on seed germination and early seedling growth of four boreal tree species. Journal of Chemical Ecology 22(5): 973–986. https://doi.org/ 10.1007/BF02029948.
Janke R.A. (1970). Transpiration resistance in Vaccinium myrtillus. American Journal of Botany 57: 1051–1054. https://doi.org/10.1002/j.1537-2197.1970.tb09908.x.
Jonsdottir I.S., Callaghan T.V. (1988). Interrelationships between different generations of interconnected tillers of Carex bigelowii. Oikos 52(1): 120–128. https://doi.org/10.2307/3565991.
Kuuluvainen T., Aakala T. (2011). Natural forests dynamics in Boreal Fennoscandia: a review and classification. Silva Fennica 45(5): 823–841. https://doi.org/10.14214/sf.73.
Lõhmus A., Remm L. (2017). Disentangling the effects of seminatural forestry on an ecosystem good: Bilberry (Vaccinium myrtillus) in Estonia. Forest Ecology and Management 404: 75–83. https://doi.org/10.1016/j.foreco.2017.08.035.
Mäkipää R. (1999). Response patterns of Vaccinium myrtillus and V. vitis-idaea along nutrient gradients in boreal forest. Journal of Vegetation Science 10: 17–26. https://doi.org/10.2307/3237156.
Miina J., Hotanen J.P., Salo K. (2009). Modelling the abundance and temporal variation in the production of bilberry (Vaccinium myrtillus L.) in Finnish mineral soil forests. Silva Fennica 43(4): 577–593. https://doi.org/10.14214/sf.181.
Nestby R., Percival D., Martinussen I., Opstad N., Rohloff J. (2011). The European blueberry (Vaccinium myrtillus L.) and the potential for cultivation. A review. The European Journal of Plant Science and Biotechnology 5: 5–16.
Nielsen A., Totland O., Ohlson M. (2007). The effect of forest management operations on population performance of Vaccinium myrtillus on a landscape scale. Basic and Applied Ecology 8(3): 231–241. https://doi.org/10.1016/j.baae.2006.05.009.
Nilsson M.C., Wardle D. (2005). Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Frontiers in Ecology and the Environment 3(8): 421–428. https://doi.org/10.1890/1540-9295(2005)003[0421:UVAAFE]2.0.CO;2.
R Core Team (2017). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Ritchie J.C. (1956). Vaccinium myrtillus L. Journal of Ecology 44: 291–299.
Rixen C., Casteller A., Schweingruber F.H., Stoeckli V. (2004). Age analysis helps to estimate plant performance on ski pistes. Botanica Helvetica 114: 127–138. https://doi.org/10.1007/s00035-004-0692-5.
Schaal B. (1978). Age structure in Liatris acidota (Compositae). Oecologia 32(1): 93–100. https://doi.org/10.1007/BF00344693.
Schweingruber F.H., Poschlod P. (2005). Growth rings in herbs and shrubs: life span, age determination and stem anatomy. Swiss Federal Research Institute WSL. 415 p.
Tolvanen A. (1995). Aboveground growth habits of two Vaccinium species in relation to habitat. Canadian Journal of Botany 73(3): 465–473. https://doi.org/10.1139/b95-047.
Wallstedt A., Gallet C., Nilsson M.-C. (2005). Behaviour and recovery of the secondary metabolite Batatasin-III from boreal forest humus: influence of temperature, humus type and microbial community. Biochemical Systematics and Ecology 33(4): 385–407. https://doi.org/10.1016/j.bse.2004.08.007.
Total of 24 references.

