Do tree cavity density and characteristics vary across topographical habitats in the tropics? A case study from Xishuangbanna, southwest China
Liu J., Tang J., Chen S.-C., Ma W., Zheng Z., Dong T. (2019). Do tree cavity density and characteristics vary across topographical habitats in the tropics? A case study from Xishuangbanna, southwest China. Silva Fennica vol. 53 no. 1 article id 10019. https://doi.org/10.14214/sf.10019
Highlights
- Cavities were significantly more abundant in high- and low-slope than high-plateau habitats
- There are more “butt hollow” cavities in high-slope habitat and they occurred at a lower height
- More “crack” cavities in low-slope habitat and they had a narrower entrance diameter
- Certain types of cavities are concentrated in specific habitats, which provide opportunities for forest management and biodiversity conservation.
Abstract
Despite the influence of cavities on the survival and distribution of cavity-dependent fauna, the variation in the density and characteristics of tree cavities across different habitat types in tropical forests is unknown. In this study, we surveyed 26 312 living trees from 376 species and compared cavity density and characteristics (height, size, type, and orientation) across five habitat types (valley, low-slope, high-slope, high-gully, and high-plateau) in a 20-hectare tropical rainforest in southwest China. From a total of 2047 cavities, we found that cavity density was mainly driven by habitat rather than tree species richness or diameter at breast height (DBH), and the characteristics of cavities were not uniformly distributed across habitats. Cavities were significantly more abundant in high- and low-slope than high-plateau habitats. Compared with other habitats, more “butt hollow” cavity types were found in high-slope habitat and they occurred at a lower tree height, whereas more “crack” cavities were found in low-slope habitat and they had a narrower entrance diameter. Although the mean orientation of cavities faced towards the northeast, cavity orientation varied significantly across habitat types. Our results indicate that certain types of cavities are concentrated in specific habitat types, which can provide avenues for forest management and biodiversity conservation. We highlight the importance of habitat heterogeneity in providing resources for cavity nesters.
Keywords
heterogeneity;
cavity-dependant animals;
tropical rainforest;
biodiversity conservation
- Liu, Key Laboratory of Southwest China Wildlife Resources Conservation of Ministry of Education, Key Laboratory of Environmental Science and Biodiversity Conservation (Sichuan Province) and Institute of Plant Adaptation and Utilization in Southwest Mountains, China West Normal University, Nanchong, Sichuan 637009, China; Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, Yunnan 666303, China E-mail liujunyan2300@163.com
- Tang, Key Laboratory of Southwest China Wildlife Resources Conservation of Ministry of Education, Key Laboratory of Environmental Science and Biodiversity Conservation (Sichuan Province) and Institute of Plant Adaptation and Utilization in Southwest Mountains, China West Normal University, Nanchong, Sichuan 637009, China E-mail jft@nn.ch
- Chen, Royal Botanic Gardens, Kew, Wakehurst Place, West Sussex RH17 6TN, UK; Mitrani Department of Desert Ecology, Swiss Institute for Dryland Environmental and Energy Research, Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Midreshet Ben-Gurion, Beer-Sheva 8499000, Israel E-mail chensichong0528@gmail.com
- Ma, Ecological Restoration and Conservation of Forests and Wetlands Key Laboratory of Sichuan Province, Sichuan Academy of Forestry, Chengdu 610081, China E-mail mawenbao_2000@126.com
- Zheng, Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, Yunnan 666303, China E-mail dioeco@outlook.com
-
Dong,
Key Laboratory of Southwest China Wildlife Resources Conservation of Ministry of Education, Key Laboratory of Environmental Science and Biodiversity Conservation (Sichuan Province) and Institute of Plant Adaptation and Utilization in Southwest Mountains, China West Normal University, Nanchong, Sichuan 637009, China
E-mail
dongtf@aliyun.com

Received 18 July 2018 Accepted 8 March 2019 Published 21 March 2019
Views 134787
Available at https://doi.org/10.14214/sf.10019 | Download PDF

Supplementary Files
1 Introduction
Tree cavities are important ecological resources in forest ecosystems because they can provide refuges or breeding sites for animals (Sedgeley 2001; Aitken and Martin 2007; Goldingay 2009; Sverdrup-Thygeson et al. 2010; Remm and Lõhmus 2011; Kikuchi et al. 2013; Kozák et al. 2018; Zhang et al. 2019). It is estimated that tree cavities are used by over 300 vertebrate species in Australia (Gibbons and Lindenmayer 2002) and by over 1700 bird species globally (van der Hoek et al. 2017). The density and characteristics of tree cavities are considered to be limiting factors for the abundance and diversity of cavity-dependent fauna (Remm et al. 2008), as different species of cavity users require different cavity characteristics for breeding and roosting (Newton 1994; Cockle et al. 2010, 2011a; Sverdrup-Thygeson et al. 2010; Davis et al. 2013). Under normal circumstances, the accessibility of cavities to predators and the internal microclimate are probably key factors for small cavity-nesting birds (Purcell et al. 1997). Consequently, cavity-nesting birds may select tree cavities at specific heights above the ground and with specific entrance diameters (Cockle et al. 2011b; Politi et al. 2010), which may restrict access to predators and increase the likelihood of nest success (Cockle et al. 2015). A specific orientation can influence the microclimate of the cavity (Inouye 1976) and is preferred by some cavity nesters. Cavity orientation can reduce energy expenditure by maintaining the inner temperature of the cavity and sealing the cavity entrance with the nester’s body when it rains or blows. Cavity type is also an important variable in nest-site selection, e.g. branch cavities were preferred by non-excavators (Wang et al. 2003; Bai et al. 2005). The diversity of cavity characteristics can enhance species richness at other trophic levels through niche partitioning. Therefore, the density and characteristics of cavities should be taken into account in biodiversity conservation and management.
It is generally believed that the density and characteristics of tree cavities are related to habitat type. As the stems of trees exposed to wind tend to break, more cavities are found in trees at high elevations and on high slopes in south-eastern Australia (Harper et al. 2005). Trees growing in higher rainfall areas and more fertile soils are more likely to produce smaller cavities (Fox et al. 2001), although they might develop cavities more slowly than those in drier forests (Koch et al. 2008a). There may also be a larger variation in the number of cavities in wet than dry forests (Gibbons and Lindenmayer 2002). These variations could be explained by different habitat attributes, such as forest stand characteristics (tree species composition and tree density), topographic position, and stochastic disturbance (Lindenmayer et al. 1993; Gibbons and Lindenmayer et al. 1997; Fan et al. 2003; Grüebler et al. 2013; McLean et al. 2015; Altamirano et al. 2017).
Studies on tree cavities in different habitats have mainly been conducted in temperate forests (Kozák et al. 2018). Compared to temperate forests, tropical forests harbour more cavity-using species worldwide (Cockle et al. 2011b) and support a variety of cavity-nesting bird species (Monterrubio-Rico and Escalante-Pliego 2006). However, the variations in cavity density and characteristics across habitat types have not been well studied in tropical areas. To our knowledge, the only study on cavity characteristics in the tropics is by Vázquez and Renton (2015), who found that compared with semi-deciduous and Piranhea forests, cavities in deciduous forests were fewer and had lower heights and smaller entrance widths, i.e. cavity density and characteristics varied significantly among vegetation types. However, their study considered vegetation types that were classified according to forest stand characteristics but not topographical habitats. Topography is strongly linked to soil water and nutrient availability at large spatial scales (Balvanera et al. 2011), and together with soil resources, it can shape local tree species composition and diversity (Baldeck et al. 2013; Liu et al. 2014; Zhang et al. 2019), which can influence cavity formation. Therefore, it is important to examine cavity density and characteristics across different habitat types that are classified based on their topographical variables in the tropics.
In the present study, we quantified the density and characteristics of tree cavities across five habitat types (valley, low-slope, high-slope, high-gully, and high-plateau) in a tropical forest in southwest China. This region is rich in biodiversity but also vulnerable owing to its latitude (northern edge of the tropics), elevation, and climate (Zhu et al. 1998). The region has been listed as a global biodiversity conservation priority area (Myers et al. 2000). In this region, a previous study found that cavity trees (trees bearing at least one cavity) have a higher abundance than those in temperate forests, which is influenced by topography (Liu et al. 2018). However, it remains unknown whether cavity characteristics (e.g. size, height, and orientation) vary across space at different topographies. In our study, the objectives were: (i) to provide reference values for cavity density and characteristics in a tropical forest, and (ii) to evaluate the importance of topographical habitats to cavity density and characteristics. We hypothesise that cavity density and characteristics vary across habitat types. Our findings can potentially be used to assist with the management of tree cavities for biodiversity conservation in tropical forests.
2 Method
2.1 Study area
Our study site was a 20-hectare forest plot (21°36´N, 101°34´E), belonging to the Center for Tropical Forest Science‒Forest Global Earth Observatory network, located in Xishuangbanna National Nature Reserve, southwest China (Supplementary file S1). The study plot was rectangular (400 m wide by 500 m long) and divided into subplots (n = 500) of 20 × 20 m, with elevations ranging from 710 to 866 m above sea level. The forest is characterised as seasonal tropical rainforest, includes 468 species of trees, and dominated by large individuals of Parashorea chinensis H. Wang (Dipterocarpaceae) (Cao et al. 2006). The annual mean temperature is 21.5 °C, the mean temperature of the coldest month (January) is 14.8 °C, and of the warmest month (June) is 25.5 °C. Annual mean precipitation is 1557 mm, but more than 87% of it occurs in the rainy season (May to October). The minimum monthly rainfall is 9.4 mm (February), which was recorded by a nearby weather station.
Following Yang et al. (2014) and Liu et al. (2014), and on the basis of the topographical variables, the study site was classified into six habitat types: Valley (slope (S) < Smean (mean slope of the plot), elevation (E) < Emean (mean elevation of the plot)): Low-slope (S ≥ Smean, E < Emean); High-slope (S ≥ Smean, E ≥ Emean, convexity > 0); High-gully (S ≥ Smean, E ≥ Emean, convexity < 0); High-plateau (S ≤ Smean, E ≥ Emean); and Forest gap (canopy cover less than 50%) (Suppl. file S2). As gap habitat was related to light availability rather than topography, forest gap plots were excluded in further analyses. The surface area and the number of plots/trees in each category are given in Suppl. file S3. The twenty most dominant species in each habitat are shown in Suppl. file S4, and the detailed topographical characteristics of the habitat types are given in Suppl. file S5.
2.2 Survey of cavities and their characteristics
A tree cavity was defined as a hole with an entrance width ≥ 2 cm and depth ≥ 2 cm, following Fan et al. (2003) and Koch et al. (2008b). The stems of all living standing trees with DBH ≥ 5 cm in our study site were scanned from the ground using binoculars (10 × 25). Potential cavities at a low height were inspected using a ladder or by climbing the tree and measured using a measuring tape. There were potential cavities at heights high above the ground, but we could not determine their suitability (≥2 cm deep) because we could not access them. In an attempt to overcome these limitations, we stood on higher ground with open views where we could observe each cavity, sometimes for long periods (once or twice for at least 2 h), to determine whether they were utilised by animals or not (Cockle et al. 2008).
Before embarking on a full-scale survey, ten trees were selected for each height class in each habitat and surveyed using ground observation and climbing methods. The proportion of actual cavities identified by the ground surveys was more than 80%. Thus, we believed that ground surveys would be an effective method for estimating cavity abundance in our study plot. Next, we recorded the number of cavities per tree, and examined and measured the cavity characteristics i.e. cavity size, height above the ground, cavity type, and orientation of each cavity. For inaccessible cavities (those high above the ground), we estimated cavity height (H) and size (S) using a measuring tape and digital dendrometer (Criterion RD 1000) to determine the angle and horizontal distance (d) between the observer and cavity, which enabled us to calculate cavity height and size using a trigonometric formula:
![]()
where α is the horizontal dip angle between centre of cavity and observer and β is the horizontal dip angle between the bottom of tree and observer.
![]()
where Htop and Hbottom are the height of top and bottom of cavity, respectively (De Labra-Hernández and Renton 2016). To improve accuracy, cavity scanning was conducted between 10:00 and 16:00 on sunny days from different directions by three surveyors.
The following characteristics were recorded for each cavity (following the methods in Lindenmayer et al. 2000; Remm et al. 2006): (1) Size: the diameter of the cavity entrance was assigned to one of five size categories, 2–5, 5–15, 15–30, 30–60, and ≥60 cm; (2) Height: the height of the cavity entrance above the ground was classified into six classes at 2-m intervals, <2, 2–4, 4–6, 6–8, 8–10, and ≥10 m; (3) Type: butt hollow (located at the basal main trunk), trunk main (located at the non-end and basal main stem), trunk top (located at the top of main trunk), crack (long, narrow cracks in the main trunk), branch middle (located in non-end and basal lateral branches), branch end (located at the end of lateral branches), and bayonet (spike-shaped cavity located at the end of lateral branches). The types of cavities were classified according the location of the cavity in the tree; a schematic figure is presented in Lindenmayer et al. (2000) and defined in Larrieu et al. (2018); and (4) Orientation: the aspect of the cavity entrance was classified as
N (>315–45°), E (>45–135°), S (>135–225°), W (>225–315°), and chimney cavities (i.e. vertical entrance; Camprodon et al. 2008).
2.3 Data analyses
The Kolmogorov-Smirnov test was applied to determine whether the data complied with a normal distribution. One-way analysis of variance (ANOVA) with least significant difference (LSD) post hoc analysis was used to compare the variation across habitats if data conformed to a normal distribution. If data deviated from a normal distribution, Kruskal-Wallis ANOVA with Dunn-Bonferroni post hoc analysis was used to compare the variation across habitat types (Zar 1999). We also applied a chi-square contingency table to compare cavity size, height, type, and orientation across habitats, and cavity type across DBH classes for the twenty dominant species. Mean cavity orientation was calculated for each habitat after excluding data on chimney cavities. A Rayleigh test for uniformity was used to assess whether the mean orientation of cavities was randomly distributed in each habitat (following formulas presented in Zhang and Yang 1992; Mao and He 2014). Statistical analyses were performed using SPSS, version 19.0.
Generalized linear mixed models (GLMMs) were used to assess the effects of local plot structure and topographical spatial variability on density and characteristics of tree cavities. Fixed effects included tree species richness (i.e. total number of tree species per subplot), RMS (root mean square or square root of the mean square) of DBH in a given subplot, and topographical spatial variability. Because density of the tree cavities is a strictly positive and continuous variable, we used GLMMs with a gamma error distribution and log link function (McCullagh and Nelder 1989). Model parameters were estimated using Laplace approximation and their significance was tested using likelihood ratio tests (Bolker et al. 2009). To compare the relative importance of the fixed effects, we calculated semi-partial marginal determination coefficients (Rm2; Nakagawa et al. 2017) derived from a commonality analysis (Ray-Mukherjee et al. 2014). All these analyses were performed in R version 3.4.3 (R Core Team 2017) using the “lme4” package (Bates et al. 2015).
3 Results
3.1 Density of cavities
We surveyed a total of 26 312 living trees from 376 species. A total of 2047 cavities were found in our study site. Cavity density varied significantly across the five habitat types (H = 20.420, df = 4, P < 0.001, Table 1). Cavities in high-slope habitat exhibited the greatest density of 129.2 cavities per hectare, and were statistically similar to those in low-slope habitat (118.1 cavities per hectare). The density of tree cavities was significantly lower in high-plateau habitat (85.3 cavities per hectare) than in high- and low-slope habitats. Similarly, the density of trees with cavities (H = 22.538, df = 4, P < 0.001) and the number of cavities per tree (H = 18.022, df = 4, P = 0.001) were both significantly different across habitats. The densities of trees with cavities and numbers of cavities per tree in low- and high-slope habitats were both significantly higher than those in high-plateau habitat, while no significant difference was found across other habitats (Table 1).
| Table 1. Mean ± SD of cavity density, density of trees with cavity and the number of cavities per tree in different habitat types in a seasonal rainforest in tropical China. Significant differences within columns (Kruskal-Wallis ANOVA with post-hoc Dunn-Bonferroni test) are character coded. | |||
| Habitat type | Cavity density (cavities per hectare) | Density of trees with cavity (trees per hectare) | No. of cavities (cavities/tree) |
| Valley | 102.3 ± 76.4 ab | 80.9 ± 53.9 bc | 0.086 ± 0.065 a |
| Low-slope | 118.1 ± 76.1 a | 95.3 ± 58.1 ab | 0.091 ± 0.057 a |
| High-slope | 129.2 ± 84.0 a | 101.7 ± 55.1 a | 0.081 ± 0.052 a |
| High-gully | 117.4 ± 87.0 ab | 89.4 ± 61.6 abc | 0.081 ± 0.058 ab |
| High-plateau | 85.3 ± 70.2 b | 70.0 ± 55.4 c | 0.063 ± 0.063 b |
| df | 4 | 4 | 4 |
| Statistic | H = 220.420 | H = 22.538 | H = 18.022 |
| P | <0.001 | <0.001 | 0.001 |
| P values <0.05 are shown in bold. | |||
3.2 Characteristics of cavities
The vast majority of cavities occurred at heights of <2 m above the ground in all habitat types, and the proportion of cavities decreased with increasing cavity height (Fig. 1a). The distribution of cavity height classes differed significantly across habitats (χ2 = 36.093, df = 20, P = 0.015; Fig. 1a). Cavity density was higher in high-slope habitat than in the other four habitat types when the height of the cavity entrance was <4 m (Table 2a). Cavity density in high-gully habitat showed significant differences compared to valley and high-plateau habitats when cavities occurred at heights of 6–8 m. However, when tree cavities were at heights of 4–6 m (H = 6.237, df = 4, P = 0.182), 8–10 m (H = 4.016, df = 4, P = 0.404), and ≥10 m (H = 4.745, df = 4, P = 0.314), no significant differences were apparent across habitats.
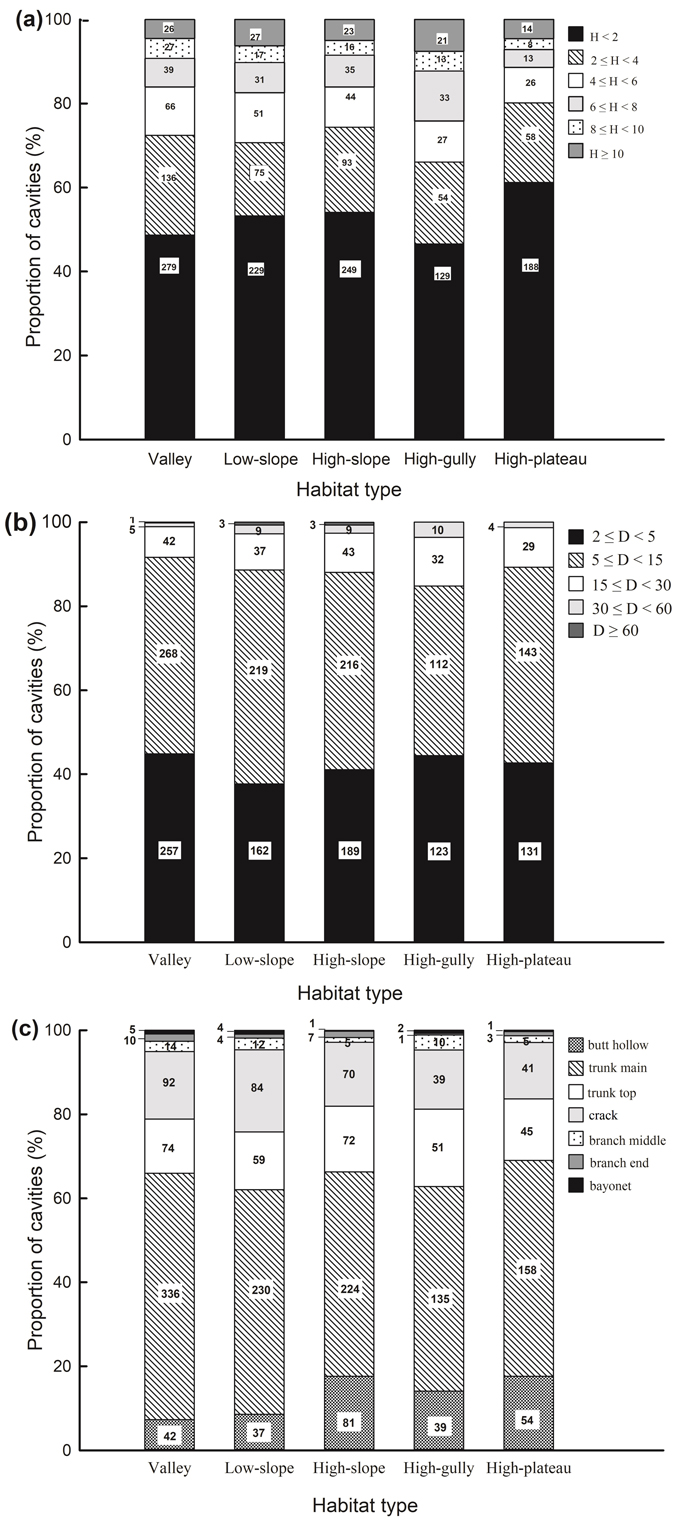
Fig. 1. Proportion of cavities with (a) cavity height above ground, (b) cavity size, (c) cavity type across different habitat types in a seasonal rainforest in tropical China. The number of cavities in each category is shown.
| Table 2. Mean ± SD of cavity density (cavities per hectare) across habitats in a seasonal rainforest in tropical China. Significant differences within rows (Kruskal-Wallis ANOVA with post-hoc Dunn-Bonferroni test) are character coded. | ||||||||
| Variable | Valley | Low-slope | High-slope | High-gully | High-plateau | df | H | P |
| (a): Cavity height | ||||||||
| <2 m | 49.8 ± 49.6 b | 62.9 ± 54.7 ab | 69.9 ± 53.9 a | 54.7 ± 53.2 ab | 52.2 ± 50.6 ab | 4 | 13.841 | 0.008 |
| 2–4 m | 24.3 ± 28.6 ab | 20.6 ± 24.9 ab | 26.1 ± 27.7 a | 22.9 ± 27.2 ab | 16.1 ± 27.4 b | 4 | 10.866 | 0.028 |
| 4–6 m | 11.8 ± 20.4 | 14.0 ± 19.4 | 12.4 ± 22.0 | 11.4 ± 20.4 | 7.2 ± 14.1 | 4 | 6.237 | 0.182 |
| 6–8 m | 7.0 ± 15.3 b | 8.5 ± 17.2 ab | 9.8 ± 17.9 ab | 14.0 ± 20.9 a | 3.6 ± 10.3 b | 4 | 17.605 | 0.001 |
| 8–10 m | 4.8 ± 14.0 | 4.7 ± 13.9 | 4.5 ± 11.7 | 5.5 ± 11.4 | 2.2 ± 7.2 | 4 | 4.016 | 0.404 |
| ≥10 m | 4.6 ± 13.3 | 7.4 ± 17.3 | 6.5 ± 17.1 | 8.9 ± 21.2 | 3.9 ± 13.5 | 4 | 4.745 | 0.314 |
| (b): Cavity size | ||||||||
| 2–5 cm | 45.9 ± 46.7 | 44.5 ± 39.4 | 53.1 ± 44.4 | 52.1 ± 47.9 | 36.4 ± 38.6 | 4 | 8.360 | 0.079 |
| 5–15 cm | 47.9 ± 46.3 ab | 60.2 ± 47.7 a | 60.7 ± 55.4 a | 47.5 ± 51.2 ab | 39.7 ± 42.9 b | 4 | 15.060 | 0.005 |
| 15–30 cm | 7.5 ± 14.6 | 10.2 ± 19.7 | 12.1 ± 22.6 | 13.6 ± 23.8 | 8.1 ± 13.9 | 4 | 3.433 | 0.488 |
| 30–60 cm | 0.9 ± 4.7 b | 2.5 ± 8.4 ab | 2.5 ± 8.5 ab | 4.2 ± 10.5 a | 1.1 ± 6.4 ab | 4 | 11.445 | 0.022 |
| ≥60 cm | 0.2 ± 2.1 | 0.8 ± 4.5 | 0.8 ± 4.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 4 | 6.970 | 0.138 |
| (c): Cavity type | ||||||||
| butt hollow | 7.5 ± 15.2 b | 10.2 ± 16.7 b | 22.8 ± 30.8 a | 16.5 ± 26.9 ab | 15.0 ± 25.4 ab | 4 | 24.231 | <0.001 |
| trunk main | 60.0 ± 48.5 ab | 63.2 ± 52.5 ab | 62.9 ± 51.1 a | 57.2 ± 45.5 ab | 43.9 ± 44.7 b | 4 | 11.271 | 0.024 |
| trunk top | 13.2 ± 21.3 | 16.2 ± 24.8 | 20.2 ± 23.2 | 21.6 ± 33.3 | 12.5 ± 18.8 | 4 | 9.724 | 0.065 |
| crack | 16.4 ± 25.2 ab | 23.1 ± 26.7 a | 19.7 ± 24.3 ab | 16.5 ± 25.3 ab | 11.4 ± 21.6 b | 4 | 15.341 | 0.004 |
| branch middle | 2.5 ± 8.1 | 3.3 ± 13.0 | 1.4 ± 8.7 | 4.2 ± 11.5 | 1.4 ± 5.8 | 4 | 6.283 | 0.179 |
| branch end | 1.8 ± 7.1 | 1.1 ± 5.2 | 2.0 ± 6.8 | 0.4 ± 3.3 | 0.8 ± 4.5 | 4 | 3.993 | 0.407 |
| bayonet | 0.9 ± 4.7 | 1.1 ± 5.2 | 0.3 ± 2.6 | 0.8 ± 4.6 | 0.3 ± 2.6 | 4 | 3.118 | 0.538 |
| P values <0.05 are shown in bold. | ||||||||
Cavity size varied from 0.3% (cavities with sizes ≥60 cm) to 46.8% (cavities with sizes of 5–15 cm), and this skewed distribution existed in every habitat (Fig. 1b). Cavity size was significantly correlated with habitat type (χ2 = 25.277, df = 16, P = 0.045). Cavities with diameters of 5–15 cm were more abundant in low- and high-slope than high-plateau habitats, and cavities with diameters of 30–60 cm were significantly more abundant in high-gully than valley habitats (Table 2b). However, cavities with other sizes (i.e. 2–5, 15–30, and ≥60 cm) were uniformly distributed across habitats.
“Trunk main” cavities were the most common type of cavity (53.3% out of all cavities), while “bayonet” cavities (0.6%) were the most scarce. This uneven distribution was detected in all habitats (Fig. 1c). Cavity types varied significantly across habitats (χ2 = 63.285, df = 24, P < 0.001; Table 2c). “Butt hollow” cavities in high-slope habitat were significantly more abundant compared to those in valley and low-slope habitats (H = 24.231, df = 4, P < 0.001). The abundance of “trunk main” cavities in high-slope habitat (H = 11.271, df = 4, P = 0.024) and “crack” cavities in low-slope habitat (H = 15.341, df = 4, P = 0.004) were significantly greater than in high-plateau habitat. However, no significant differences were found for other cavity types across habitats. Cavity types differed significantly across the twenty dominant species, across all DBH classes, and within each DBH class (except for DBH 40–60 and ≥60 cm because of the sparsity of individuals in these classes, Suppl. file S6).
There was a significant difference in the orientation of cavity entrances across habitats (χ2 = 45.152, df = 16, P < 0.001). The orientation of cavity entrances in low-slope habitat was more likely to face towards the east, while cavities in high-gully and high-plateau habitats were more likely to face north (Table 3). The orientations of cavities in valley and high-slope habitats did not differ significantly.
| Table 3. Rayleigh test of orientation of cavities in each habitat in a seasonal rainforest in tropical China. | |||||
| Habitat | No. of cavities | Mean orientation | Z-value | P | 95% confidence intervals |
| Valley | 474 | 95.8° | 0.050 | 0.951 | – |
| Low-slope | 342 | 110.9° | 3.651 | 0.026 | 110.9 ± 47.4° |
| High-slope | 388 | 49.4° | 1.711 | 0.181 | – |
| High-gully | 218 | 327.7° | 3.604 | 0.027 | 327.7 ± 51.7° |
| High-plateau | 229 | 341.7° | 4.078 | 0.017 | 341.7 ± 44.3° |
| P values <0.05 are shown in bold. | |||||
3.3 Key factors affecting tree cavity densities
The total density of tree cavities was significantly correlated with tree species richness, RMS DBH, and topographical spatial variability, however, there was a higher likelihood ratio test statistic (χ2) with topographical spatial variability (133.937) than with tree species richness (43.405) or RMS DBH (20.029) (Table 4).
| Table 4. Summary of generalized linear mixed models relating density of cavity types to fixed effects. Likelihood ratio test statistics (χ2), probabilities (P), semi-partial marginal determination coefficients (Rm2 [%]) are displayed. Model parameters were considered significant at 5% and are highlighted in bold. | |||||||||||
| Model | Tree species richness | RMS DBH | Topographical spatial variability | ||||||||
| χ2 | P | Rm2 (%) | χ2 | P | Rm2 (%) | χ2 | P | Rm2 (%) | |||
| All density | 43.405 | <0.001 | 4.127 | 20.029 | <0.001 | 1.092 | 133.937 | <0.001 | 22.57 | ||
| Density | |||||||||||
| Cavity height | |||||||||||
| <2 m | 33.484 | <0.001 | 3.325 | 6.807 | 0.009 | 0.442 | 142.781 | <0.001 | 24 | ||
| 2–4 m | 10.833 | 0.001 | 2.702 | 10.922 | 0.001 | 3.437 | 59.892 | <0.001 | 14.927 | ||
| 4–6 m | 2.434 | 0.119 | 0.8 | 0.631 | 0.427 | 0.212 | 17.582 | <0.001 | 9.023 | ||
| 6–8 m | 0.787 | 0.375 | 0.437 | 0.23 | 0.632 | 0.124 | 43.973 | <0.001 | 27.256 | ||
| 8–10 m | 0.032 | 0.857 | 0.164 | 0.059 | 0.809 | 0 | 9.046 | 0.003 | 8.544 | ||
| ≥10 m | 0.447 | 0.504 | 0.251 | 0.194 | 0.659 | 2.072 | 66.813 | <0.001 | 56.919 | ||
| Cavity size | |||||||||||
| 2–5 cm | 4.911 | 0.027 | 1.161 | 1.19 | 0.275 | 0.214 | 136.22 | <0.001 | 29.075 | ||
| 5–15 cm | 23.278 | <0.001 | 2.828 | 6.374 | 0.012 | 0.359 | 74.336 | <0.001 | 14.437 | ||
| 15–30 cm | 13.004 | <0.001 | 3.634 | 15.984 | <0.001 | 1.987 | 50.465 | <0.001 | 21.799 | ||
| 30–60 cm | 4.617 | 0.032 | 3.326 | 1.602 | 0.206 | 0.493 | 19.456 | <0.001 | 34.47 | ||
| Cavity type | |||||||||||
| Butt hollow | 18.32 | <0.001 | 4.863 | 3.036 | 0.081 | 0.068 | 39.474 | <0.001 | 15.029 | ||
| Trunk main | 11.469 | 0.001 | 1.978 | 0.098 | 0.754 | 0.012 | 91.989 | <0.001 | 18.33 | ||
| Trunk top | 23.692 | <0.001 | 4.062 | 0.673 | 0.412 | 0.818 | 43.873 | <0.001 | 14.039 | ||
| Crack | 5.898 | 0.015 | 0.349 | 5.561 | 0.018 | 0.529 | 35.16 | <0.001 | 11.306 | ||
| Branch middle | 18.724 | <0.001 | 0.391 | 14.611 | 0 | 1.401 | 51.162 | 0.001 | 55.476 | ||
| Branch end | 5.412 | 0.02 | 4.444 | 2.02 | 0.155 | 4.951 | 12.815 | 0.541 | 31.914 | ||
| RMS DBH, root mean square diameter of trees at breast height in a given subplot. | |||||||||||
Topographical spatial variability was significantly correlated with cavity height, size, and type (except “branch end”). Tree species richness was significantly correlated with the density of cavity height <4 m and cavity size 5–60 cm and RMS DBH was significantly correlated with the density of cavity height <4 m, cavity size 5–30 cm, and “branch middle” cavity type. Overall, the effects of tree species richness or RMS DBH on the density of cavity characteristics were far less than that of topographical spatial variability (Table 3).
4 Discussion
In the present study, we performed the first quantitative tree cavity analyses and comparison of cavity characteristics (height, size, type, and orientation) in different topographical habitat types in Xishuangbanna–a typical tropical (northern edge of the tropics) forest. Our results indicated that habitat types showed a variation in the distribution of cavities in terms of density and characteristics in the region. Firstly, the density of cavities in the study site was very high, and higher than in other reported studies (Boyle et al. 2008; Remm and Lõhmus 2011); and secondly, the distribution of height, size, cavity type, and orientation of cavity entrances varied across habitat types. Xishuangbanna is one of the most biodiverse regions in the world and is designated as a global hotspot for biodiversity (Myers et al. 2000). Thus, our findings can provide new insights for local biodiversity conservation.
4.1 Density of cavities
Previous studies found that the density of cavities in tropical forests varied widely from 0.5 to 407 cavities per hectare (Pattanavibool and Edge 1996; Stoneman et al. 1997; Renton 2004; Boyle et al. 2008); therefore, the densities in our study (85.3–129.2 cavities per hectare across habitats) are among the highest reported and are far higher than the global median density (16 cavities per hectare) (Remm and Lõhmus 2011). The high density of cavities in high-slope habitat is probably due to the high density of trees occurring there (Suppl. file S4). In accordance with the findings in other forests (Temesgen et al. 2008; Zheng et al. 2009), a high density of trees provides greater opportunities for tree-fall (especially on the high slopes) resulting in cavity formations in neighbouring trees (usually cracked by a fallen neighbour individual), which could explain the high density of cavities found in high-slope habitat in our study. Additionally, topography may be a factor influencing cavity density. Liu’s et al. (2018) study supports our findings, as they found that cavity occurrence was influenced by topographical factors and decreased from valleys to ridges in the same study site. The higher cavity densities in low- and high-slope than high-plateau habitats may be because exposure to wind on the slopes increases the probability of stems breaking and therefore accelerates cavity formation (Lindenmayer et al. 1993; Harper et al. 2005).
4.2 Characteristics of cavities
In our study, we found that the vast majority of cavities occurred at heights <2 m above ground level. This may be because cavities high above the ground were overlooked during surveys despite the precautions taken. The distribution of cavity height classes across habitats was mainly owing to the difference in the topography of each habitat. Trees on higher slopes had relatively lower clear bole height (Kong et al. 2014), which may result in the formation of cavities at lower heights when trees cracked frequently and branches snapped. In addition, topography is strongly linked to soil moisture and nutrient availability (Balvanera et al. 2011), e.g. sites near valleys were moister and nutritionally richer than sites near ridge tops (Gibbons and Newbery 2003; Segura et al. 2003). Thus, trees growing on sites near ridge tops usually have reduced tree heights (Carrasco et al. 2015; Dong et al. 2016). Under similar conditions, cavities in trees that are shorter may have a lower cavity height. Previous studies conducted in our study area found that soil moisture and nutrient content decreased from the valley to high-slope habitat (Yan and Cao 2008) and the maximum tree height decreased at higher elevations and steeper slopes (Liu et al. 2014). This may explain why there are more cavities at heights of <4 m in high-slope habitat compared to other habitats.
Differences in cavity types across habitats are also due to differences in the topography of each habitat. Topography typically shapes tree species composition and diversity (Liu et al. 2014), and cavity types often vary among different tree species (Lindenmayer et al. 2000; present study). Our results showed that the primary driver of cavity type density was the topographical spatial variability of the habitat rather than tree species richness. Topographical spatial variability, caused by topography, may result in specific tree species growing in specific habitats. For example, Castanopsis echinocarpa Hook.f. et Thoms. ex Miq., C. hystrix Miq., and Alseodaphne petiolaris (Meisn.) Hook. f. were mainly distributed in high-slope habitat, while species such as Baccaurea ramiflora Lour. and Mezzettiopsis creaghii Ridl. had non-random distributions in low-slope habitat, which was supported by the fact that elevation and convexity were the main drivers of species distribution patterns in the study area (Liu et al. 2014). Many of these species (e.g. B. ramiflora, C. hystrix, and M. creaghii) had high cavity occurrences (Liu et al. 2018). According to our survey, the species in high-slope habitat (mentioned above) were highly prone to form “butt hollow” cavity types than the other tree species, resulting in a higher density of “butt hollows” in high-slope than in valley and low-slope habitats. Similarly, those species in low-slope habitat were characterised by more “crack” cavities than those species in high-plateau habitat. Rather, topographical differences in habitat type may be related to differences in growth form and morphology between the various species of trees (Wormington et al. 2003; Kikuchi et al. 2013) that, in turn, affect the type of cavities that develop in trees (Lindenmayer et al. 2000). In our study, a greater re-sprouting capacity was obvious in the Fagaceae species that dominate in high-slope habitat and was responsible for the large number of “butt hollow” cavities. On the other hand, animal excavations that dominate in a specific habitat type also affected the type of cavities occurring. In our study area, a small quantity of cavities (nearly 2% of all cavities) had root-buttress concavity, which resulted in a supplement of “butt hollows” (Larrieu et al. 2018). Due to buttresses being prevalent in valley and low-slope habitats, we found that more than half of the “butt hollow” cavities were root-buttress concavities in these habitats. We found that cavity size was significantly correlated with habitat type because cavity size was obviously correlated with tree size that could differ with topography. However, the difference in RMS DBH (16.6–20.5 cm) in each habitat was negligible although it varied significantly across habitats (Suppl. file S5). This may be affected by micro-scale factors within different parts of a tree because we find no significant evidence for the correlation between topography and cavity size, which is consistent with Lindenmayer et al. (2000). In our study plot, we found that the majority of cavities had originated from fallen branches, which is in accordance with the findings in other forests (Gibbons et al. 2002; Cockle et al. 2011b; Koch et al. 2008a). Thus, the size of the branches in which cavities will form will limit the size of the cavities that develop from snapped-off branches; large branches that break at the base of the branch may leave large trunk hollows, whereas smaller cavities may occur if a branch breaks halfway along its stem.
We found that the mean orientation of cavity entrances in the five habitat types were all facing northeast, which is similar to other studies (Harper et al. 2005; Murphy and Legge 2007). This may have been influenced by the prevailing south-westerly winds in our study area. The majority of cavities in the study area were rot holes rather than woodpecker breeding cavities, which is similar to that found in other tropical forests (Boyle et al. 2008; Cockle et al. 2011b; Vázquez and Renton 2015). Therefore, it appears as if the mean orientation of cavities was mainly influenced by wind direction and may be an indirect consequence of the angle of the limbs or branches exposed to wind. Nonetheless, subtle differences in orientation were detected across habitats, suggesting that the local topography of each habitat is a possible local driver of orientation diversity. Due to topographical effects, the mean orientations of cavities in the low-slope, high-gully, and high-plateau habitats were different, but the mean aspect was consistent in each habitat. Furthermore, stochastic events such as severe storms, rodent nibbles, and woodpecker excavations also contributed to the formation of cavities with random orientations. These factors may be the reasons for the mean orientations of cavities in valley and high-slope habitats not being significantly different.
4.3 Implications for cavity-dependent fauna
Cavities are not homogeneously distributed across habitats but exhibit a pattern of high spatial aggregation, such as the cavity density being the highest in high-slope habitat in our study area. This pattern may restrict the distribution of cavity-dependent fauna, especially for secondary cavity-nesting birds, as these species are constrained by the distribution of existing cavities as nest resources (Eberhard 2002; Sverdrup-Thygeson et al. 2010). In addition, the significant variation in cavity characteristics across different topographical habitats highlights the importance of topographical diversity in providing nesting resources to a variety of cavity-dependent fauna as different species have different cavity requirements with varying characteristics (reviewed by Gibbons and Lindenmayer 1997). Therefore, our findings can assist with providing avenues for forest management and biodiversity conservation. Priority should be given to retaining diverse habitat types that support multiple cavities with a variety of characteristics (e.g. entrance sizes, heights, types, and orientations) that will benefit many cavity-dependent animals (Gibbons and Lindenmayer 2002; Le Roux et al. 2016). Meanwhile, we suggest that forest management strategies are developed for conserving the diversity of tree species to meet the requirements of different cavity-using species. Additionally, conservation management should focus on protecting large trees because they generally bear larger sized cavities and in greater quantities that could be more suitable for cavity-dwelling birds and mammals. We recommend that future studies investigate the relationships between cavity characteristics and cavity occupancy by an array of animal guilds, which could assist forest managers with revising the current guidelines for biodiversity conservation in tropical forests.
Acknowledgements
The work was supported by Sichuan Science and Technology Program (No: 2019YFS0464) and the Project of Education Department of Sichuan Province, China (No: 17AZ0374). We thank the Xishuangbanna Station of Tropical Rainforest Ecosystem Studies for providing the tree species inventory and topographic data.
References
Aitken K.E.H., Martin K. (2007). The importance of excavators in hole-nesting communities: availability and use of natural tree holes in old mixed forest in western Canada. Journal of Ornithology 148(Suppl. 2): 425–434. https://doi.org/10.1007/s10336-007-0166-9.
Altamirano T.A., Ibarra J.T., Martin K., Bonacic C. (2017). The conservation value of tree decay processes as a key driver structuring tree cavity nest webs in South American temperate rainforests. Biodiversity and Conservation 26(10): 2453–2472. https://doi.org/10.1007/s10531-017-1369-x.
Bai M.L., Wichmann F., Mühlenberg M. (2005). Nest-site characteristics of hole-nesting birds in a primeval boreal forest of Mongolia. Acta Ornithologica 40(1): 1–14. https://doi.org/10.3161/068.040.0105.
Baldeck C.A., Harms K.E., Yavitt J.B., John R., Turner B.L., Valencia R., Navarrete H., Davies S.J., Chuyong G.B., Kenfack D., Thomas D.W., Madawala S., Gunatilleke N., Gunatilleke S., Bunyavejchewin S., Kiratiprayoon S., Yaacob A., Supardi M.N., Dalling J.W. (2013). Soil resources and topography shape local tree community structure in tropical forests. Proceedings of the Royal Society B 280(1766): 20130548. https://doi.org/10.1098/rspb.2013.0548.
Balvanera P., Quijas S., Perez-Jimenez A. (2011). Distribution patterns of tropical dry forest trees along a mesoscale water availability gradient. Biotropica 43(4): 414–422. https://doi.org/10.1111/j.1744-7429.2010.00712.x.
Bates D., Machler M., Bolker B.M., Walker S.C. (2015). Fitting Linear Mixed-Effects Models using lme4. Journal of Statistical Software 67(1): 1–48. https://doi.org/10.18637/jss.v067.i01.
Bolker B.M., Brooks M.E., Clark C.J., Geange S.W., Poulsen J.R., Stevens M.H.H., White J. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution 24(3): 127–135. https://doi.org/10.1016/j.tree.2008.10.008.
Boyle W.A., Ganong C.N., Clark D.B., Hast M.A. (2008). Density, distribution and attributes of tree cavities in an old-growth tropical rain forest. Biotropica 40(2): 241–245. https://doi.org/10.1111/j.1744-7429.2007.00357.x.
Camprodon J., Salvanyà J., Solerzurita J. (2008). The Abundance and Suitability of tree cavities and their impact on hole-nesting bird populations in beech forests of NE Iberian Peninsula. Acta Ornithologica 43(1): 17–31. https://doi.org/10.3161/000164508X345293.
Cao M., Zou X., Warren M., Zhu H. (2006). Tropical forests of Xishuangbanna, China. Biotropica 38(3): 306–309. https://doi.org/10.1111/j.1744-7429.2006.00146.x.
Carrasco L.O., Bucci S.J., Francescantonio D.D., Lezcano O.A., Campanello P.L., Scholz F.G., Rodríguez S., Madanes N., Cristiano P.M., Hao G.Y., Holbrook N.M., Goldstein G. (2015). Water storage dynamics in the main stem of subtropical tree species differing in wood density, growth rate and life history traits. Tree Physiology 35(4): 354–365. https://doi.org/10.1093/treephys/tpu087.
Cockle K.L., Martin K., Wiebe K. (2008). Availability of cavities for nesting birds in the Atlantic Forest, Argentina. Ornithologia Neotropical 19(Suppl.): 269–278.
Cockle K.L., Martin K., Drever M.C. (2010). Supply of tree-holes limits nest density of cavity-nesting birds in primary and logged subtropical Atlantic forest. Biological Conservation 143(11): 2851–2857. https://doi.org/10.1016/j.biocon.2010.08.002.
Cockle K.L., Martin K., Wiebe K. (2011a). Selection of nest trees by cavity-nesting birds in the Neotropical Atlantic forest. Biotropica 43(2): 228–236. https://doi.org/10.1111/j.1744-7429.2010.00661.x.
Cockle K.L., Martin K., Wesolowski T. (2011b). Woodpeckers, decay, and the future of cavity-nesting vertebrate communities worldwide. Frontiers in Ecology and the Environment 9(7): 377–382. https://doi.org/10.1890/110013.
Cockle K.L., Bodrati A., Lammertink M., Martin K. (2015). Cavity characteristics, but not habitat, influence nest survival of cavity-nesting birds along a gradient of human impact in the subtropical Atlantic forest. Biological Conservation 184: 193–200. https://doi.org/10.1016/j.biocon.2015.01.026.
Davis A., Major R.E., Taylor C.E. (2013). Housing shortages in urban regions: aggressive interactions at tree hollows in forest remnants. Plos One 8(3): e59332. https://doi.org/10.1371/journal.pone.0059332.
De Labra-Hernández M.Á., Renton K. (2016). Importance of large, old primary forest trees in nest-site selection by the Northern Mealy Amazon (Amazona guatemalae). Tropical Conservation Science 9(4): 1–10. https://doi.org/10.1177/1940082916680361.
Dong T., Duan B., Zhang S., Korpelainen H., Niinemets Ü., Li C. (2016). Growth, biomass allocation and photosynthetic responses are related to intensity of root severance and soil moisture conditions in the plantation tree Cunninghamia lanceolata. Tree Physiology 36(7): 807–817. https://doi.org/10.1093/treephys/tpw025.
Eberhard J.R. (2002). Cavity adoption and the evolution of coloniality in cavity-nesting birds. Condor 104: 240–247.
Fan Z., Shifley S.R., Spetich M.A., Thompson F.R.III., Larsen D.R. (2003). Distribution of cavity trees in midwestern old-growth and second-growth forests. Canadian Journal of Forest Research 33(8): 1481–1494. https://doi.org/10.1139/x03-068.
Fox J.C., Burgman M.A., Ades P.K. (2001). Predictive models of hollow incidence for State Forests in central and eastern Victoria. In: Report for the Department of Natural Resources and Environment, Melbourne, Australia.
Gibbons J.M., Newbery D.M. (2003). Drought avoidance and the effect of local topography on trees in the understorey of Bornean lowland rain forest. Plant Ecology 164(1): 1–18. https://doi.org/10.1023/A:1021210532510.
Gibbons P., Lindenmayer D.B. (1997). Conserving hollow-dependent fauna in timber-production forests. New South Wales National Park and Wildlife Service Environmental Heritage Monograph Series 3: 1–110.
Gibbons P., Lindenmayer D.B. (2002). Tree hollows and wildlife conservation in Australia. CSIRO Publishing, Collingwood, Victoria, Australia. https://doi.org/10.1071/9780643090033.
Gibbons P., Lindenmayer D.B., Barry S.C., Tanton M.T. (2002). Hollow selection by vertebrate fauna in forests of southeastern Australia and implications for forest management. Biological Conservation 103(1): 1–12. https://doi.org/10.1016/S0006-3207(01)00109-4.
Goldingay R.L. (2009). Characteristics of tree hollows used by Australian birds and bats. Wildlife Research 36(5): 394–409. https://doi.org/10.1071/WR08172.
Grüebler M.U., Schaller S., Keil H., Naef-Daenzer B. (2013). The occurrence of cavities in fruit trees: effects of tree age and management on biodiversity in traditional European orchards. Biodiversity and Conservation 22(13–14): 3233–3246. https://doi.org/10.1007/s10531-013-0581-6.
Harper M.J., McCarthy M.A., van der Ree R. (2005). The abundance of hollow-bearing trees in urban dry sclerophyll forest and the effect of wind on hollow development. Biological Conservation 122(2): 181–192. https://doi.org/10.1016/j.biocon.2004.07.003.
Inouye D.W. (1976). Nonrandom orientation of entrance holes to woodpecker nests in aspen trees. Condor 78(1): 101–102. https://doi.org/10.2307/1366924.
Kikuchi K., Akasaka T., Yamaura Y., Nakamura F. (2013). Abundance and use of cavity trees at the tree- and stand-levels in natural and plantation forests in Hokkaido, Japan. Journal of Forest Research 18(5): 389–397. https://doi.org/10.1007/s10310-012-0358-x.
Koch A., Munks S., Driscoll D. (2008b). The use of hollow-bearing trees by vertebrate fauna in wet and dry Eucalyptus obliqua forest, Tasmania. Wildlife Research 35(8): 727–746. https://doi.org/10.1071/WR08007.
Koch A.J., Munks S.A., Driscoll D., Kirkpatrick J.B. (2008a). Does hollow occurrence vary with forest type? A case study in wet and dry Eucalyptus oblique forest. Forest Ecology and Management 255(12): 3938–3951. https://doi.org/10.1016/j.foreco.2008.03.025.
Kong L., Chen X., Lu S., Li S., Chen B., Gao C., Shi Y., Yang X. (2014). Relationships among growth of Larix Principis-rupprechtii, herbaceous plants diversity and landform. Bull Soil and Water Conservation 34: 60–66. [In Chinese].
Kozák D., Mikoláš M., Svitok M., Bače R., Paillet Y., Larrieu L., Nagel T.A., Begovič K., Čada V., Diku A., Frankovič M., Janda P., Kameniar O., Keren S., Kjučukov P., Lábusová J., Langbehn T., Málek J., Mikac S., Morrissey R.C., Nováková M.H., Schurrman J.S., Svobodová K., Synek M., Teodosiu M., Toromani E., Trotsiuk V., Vítková L., Svoboda M. (2018). Profile of tree-related microhabitats in European primary beech-dominated forests. Forest Ecology and Management 429: 363–374. https://doi.org/10.1016/j.foreco.2018.07.021.
Larrieu L., Paillet Y., Winter S., Bütler R., Kraus D., Krumm F., Lachat T., Michel A.K., Regnery B., Vandekerkhove K. (2018). Tree related microhabitats in temperate and Mediterranean European forests: a hierarchical typology for inventory standardization. Ecological Indicators 84: 194–207. https://doi.org/10.1016/j.ecolind.2017.08.051.
Le Roux D.S., Ikin K., Lindenmayer D.B., Bistricer G., Manning A.D. (2016). Effects of entrance size, tree size and landscape context on nest box occupancy: considerations for management and biodiversity offsets. Forest Ecology and Management 366: 135–142. https://doi.org/10.1016/j.foreco.2016.02.017.
Lindenmayer D.B., Cunningham R.B., Donnelly C.F., Tanton M.T., Nix H.A. (1993). The abundance and development of cavities in Eucalyptus trees: a case study in the montane forests of Victoria, southeastern Australia. Forest Ecology and Management 60(1–2): 77–104. https://doi.org/10.1016/0378-1127(93)90024-H.
Lindenmayer D.B., Cunningham R.B., Pope M.L., Gibbons P., Donnelly C.F. (2000). Cavity sizes and types in Australian eucalypts from wet and dry forest types-a simple of rule of thumb for estimating size and number of cavities. Forest Ecology and Management 137(1–3): 139–150. https://doi.org/10.1016/S0378-1127(99)00322-9.
Liu J., Tan T., Slik J.W.F. (2014). Topography related habitat associations of tree species traits, composition and diversity in a Chinese tropical forest. Forest Ecology and Management 330: 75–81. https://doi.org/10.1016/j.foreco.2014.06.045.
Liu J., Zheng Z., Xu X., Dong T., Chen S. (2018). Abundance and distribution of cavity trees and the effect of topography on cavity presence in a tropical rainforest, southwestern China. Canadian Journal of Forest Research 48(9): 1058–1066. https://doi.org/10.1139/cjfr-2018-0044.
Mao L., He C. (2014). Application of concentration ratio and circular distribution method to analyze the seasonal characteristics of infectious diseases. Chinese Journal of Health Statistics 31: 251–253. [In Chinese]
McCullagh P., Nelder J.A. (1989). Generalized linear models. 2nd ed. Chapman and Hall/CRC, USA.
McLean C.M., Bradstock R., Price O., Kavanagh R.P. (2015). Tree hollows and forest stand structure in Australian warm temperate Eucalyptus forests are adversely affected by logging more than wildfire. Forest Ecology and Management 341: 37–44. https://doi.org/10.1016/j.foreco.2014.12.023.
Monterrubio-Rico T.C., Escalante-Pliego P. (2006). Richness, distribution and conservation of cavity nesting birds in Mexico. Biological Conservation 128(1): 67–78. https://doi.org/10.1016/j.biocon.2005.09.017.
Murphy S.A., Legge S.M. (2007). The gradual loss and episodic creation of palm cockatoo (Probosciger aterrimus) nest-trees in a fire- and cyclone-prone habitat. Emu 107(1): 1–6. https://doi.org/10.1071/MU06012.
Myers N., Mittermeier R.A., Mittermeier C.G., Da F.G., Kent J. (2000). Biodiversity hotspots for conservation priorities. Nature 403: 853–858. https://doi.org/10.1038/35002501.
Nakagawa S., Johnson P.C.D., Schielzeth H. (2017). The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. Journal of the Royal Society Interface 14(134): 20170213. https://doi.org/10.1098/rsif.2017.0213.
Newton I. (1994). The role of nest sites in limiting the numbers of hole-nesting birds: a review. Biological Conservation 70(3): 265–276. https://doi.org/10.1016/0006-3207(94)90172-4.
Pattanavibool A., Edge W.D. (1996). Single-tree selection silviculture affects cavity resources in mixed deciduous forests in Thailand. Journal of Wildlife Management 60(1): 67–73. https://doi.org/10.2307/3802041.
Politi N., Hunter M.J, Rivera L. (2010). Availability of cavities for avian cavity nesters in selectively logged subtropical montane forests of the Andes. Forest Ecology and Management 260(5): 893–906. https://doi.org/10.1016/j.foreco.2010.06.009.
Purcell K.L., Verner J., Oring L.W. (1997). A comparison of the breeding ecology of bird nesting in boxes and tree cavities. The Auk: Ornithological Advances 114(4): 646–656. https://doi.org/10.2307/4089284.
R Core Team (2017). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Ray-Mukherjee J., Nimon K., Mukherjee S., Morris D.W., Slotow R., Hamer M. (2014). Using commonality analysis in multiple regressions: a tool to decompose regression effects in the face of multicollinearity. Methods in Ecology and Evolution 5(4): 320–328. https://doi.org/10.1111/2041-210X.12166.
Remm J., Lõhmus A. (2011). Tree cavities in forests – the broad distribution pattern of a keystone structure for biodiversity. Forest Ecology and Management 262(4): 579–585. https://doi.org/10.1016/j.foreco.2011.04.028.
Remm J., Lõhmus A., Remm K. (2006). Tree cavities in riverine forests: what determines their occurrence and use by hole-nesting passerines? Forest Ecology and Management 221(1–3): 267–277. https://doi.org/10.1016/j.foreco.2005.10.015.
Remm J., Lõhmus A., Rosenvald R. (2008). Density and diversity of hole-nesting passerines: dependence on the characteristics of cavities. Acta Ornithologica 43(1): 83–91. https://doi.org/10.3161/000164508X345365.
Renton K. (2004). Agonistic interactions of nesting and non-breeding macaws. Condor 106(2): 354–362. https://doi.org/10.1650/7388.
Sedgeley J.A. (2001). Quality of cavity microclimate as a factor influencing selection of maternity roosts by a tree-dwelling bat, Chalinolobus tuberculatus, in New Zealand. Journal of Applied Ecology 38(2): 425–438. https://doi.org/10.1046/j.1365-2664.2001.00607.x.
Segura G., Balvanera P., Duran E., Perez A. (2003). Tree community structure and stem mortality along a water availability gradient in a Mexican tropical dry forest. Plant Ecology 169(2): 259–271. https://doi.org/10.1023/A:1026029122077.
Stoneman G.L., Rayner M.E., Bradshaw F.J. (1997). Size and age parameters of nest trees used by four species of parrot and one species of cockatoo in south-west Australia: critique. Emu 97(1): 94–96. https://doi.org/10.1071/MU97012.
Sverdrup-Thygeson A., Skarpaas O., Ødegaard F. (2010). Hollow oaks and beetle conservation: the significance of the surroundings. Biodiversity and Conservation 19(3): 837–852. https://doi.org/10.1007/s10531-009-9739-7.
Temesgen H., Barrett T.M., Latta G. (2008). Estimating cavity tree abundance using Nearest Neighbor Imputation methods for western Oregon and Washington forests. Silva Fennica 4(3)2: 337–354. https://doi.org/10.14214/sf.241.
van der Hoek Y., Gaona G.V., Martin K. (2017). The diversity, distribution and conservation status of the tree-cavity-nesting birds of the world. Diversity and Distributions 23(10): 1120–1131. https://doi.org/10.1111/ddi.12601.
Vázquez L., Renton K. (2015). High density of tree-cavities and snags in tropical dry forest of western mexico raises questions for a latitudinal gradient. Plos One 10(1): e0116745. https://doi.org/10.1371/journal.pone.0116745.
Wang H., Gao W., Wan D., Liu D., Deng W. (2003). Nest-site characteristics and reproductive success of five species of birds breeding in natural cavities. Acta Ecologica Sinica 23: 1377–1385.
Wormington K.R., Lamb D., Mccallum H.I., Moloney D.J. (2003). The characteristics of six species of living hollow-bearing trees and their importance for arboreal marsupials in the dry Sclerophyll forests of southeast Queensland, Australia. Forest Ecology and Management 182: 75–92. https://doi.org/10.1016/S0378-1127(03)00010-0.
Yan X., Cao M. (2008). Seedling growth and survival of the endangered tree species Shorea wanitianshuea after a mast fruiting. Chinese Journal of Plant Ecology 32: 55–64. [In Chinese].
Yang J., Zhang G., Ci X., Swenson N.G., Cao M., Sha L., Li J., Baskin C.C., Slik J.W.F., Lin L. (2014). Functional and phylogenetic assembly in a Chinese tropical tree community across size classes, spatial scales and habitats. Functional Ecology 28(2): 520–529. https://doi.org/10.1111/1365-2435.12176.
Zar J.H. (1999). Biostatistical analysis. 4th edition. Prentice Hall. U.S.A.
Zhang F., Yang S. (1992). A formula for estimating confidence interval of the mean direction. Chinese Journal of Health Statistics 2: 20–23. [In Chinese].
Zhang R., Liu J., Liu Q., He H., Xu X., Dong T. (2019). Sexual differences in growth and defence of Populus yunnanensis under drought stress. Canadian Journal of Forest Research. https://doi.org/10.1139/cjfr-2018-0270.
Zheng Z., Zhang S.B., Yang G.P., Tang Y., Baskin J., Baskin C., Yang L.Y. (2009). Abundance and distribution of cavity trees in an old-growth subtropical montane evergreen broadleaved forest. Canadian Journal of Forest Research 39(11): 2234–2235. https://doi.org/10.1139/X09-149.
Zhu H., Wang H., Li B., Xu Z. (1998). Research on the tropical seasonal rainforest of Xishuangbanna, South Yunnan. Guihaia 18: 371–384. [In Chinese].
Total of 74 references.

