Local adaptation of phenotypic stem traits distinguishes two provenance regions of silver birch in Latvia
Gailis A., Zeltiņš P., Matisons R., Purviņš A., Augustovs J., Vīndedzis V., Jansons Ā. (2021). Local adaptation of phenotypic stem traits distinguishes two provenance regions of silver birch in Latvia. Silva Fennica vol. 55 no. 2 article id 10524. https://doi.org/10.14214/sf.10524
Highlights
- Two provenance subregions in Latvia – coastal and inland – were distinguished
- Silver birch populations in inland region possessed better growth, higher heritability, and phenotypic plasticity
- Moderate to high heritability for stem quality was estimated in both regions
- Silver birch from inland region possesses higher potential for improvement of adaptability.
Abstract
Populations of tree species with a wide geographic range, such as silver birch (Betula pendula Roth), show genetic specialization to native environments, while maintaining high phenotypical plasticity. Accordingly, assessment of local specialization is essential for adaptive management. The aim of the study was to detect geographic patterns of local adaptation of growth and stem quality based on two open-pollinated progeny trials in Latvia testing local material. Two provenance regions differing by continentality were distinguished, which also differed in genetic control of growth traits, likely originating from the post-glacial recolonization of vegetation and subsequent natural adaptation. Heritability of the traits was estimated for each of the distinguished regions, indicating differing patterns of genetic adaptation and potential for future selection. Trees from the more continental inland showed superior growth and possessed higher heritability. The coastal provenance region showed slower growth and intermediate heritability of the respective traits. Moderate to high heritability for stem quality traits was estimated irrespectively of region. Overall, better growth and higher heritability suggests that anthropogenic selection within the best inland provenances may constitute better performing and adaptable breeding population compared to the coastal one. Still, overlapping phenotypical variation and heritability of quality traits implies improved stemwood quality for plywood regardless of the provenance region. High adaptive capacity of silver birch genotypes suggests ability to cope with climatic changes, highlighting its potential for climate-smart forestry.
Keywords
Betula pendula;
population genetics;
genetic parameters;
climate-smart forestry;
phenotypic traits
- Gailis, Latvian State Forest Research Institute Silava, 111 Rigas street, LV-2169, Salaspils, Latvia E-mail arnis.gailis@silava.lv
-
Zeltiņš,
Latvian State Forest Research Institute Silava, 111 Rigas street, LV-2169, Salaspils, Latvia
E-mail
pauls.zeltins@silava.lv

-
Matisons,
Latvian State Forest Research Institute Silava, 111 Rigas street, LV-2169, Salaspils, Latvia
 https://orcid.org/0000-0002-4042-0689
E-mail
roberts.matisons@silava.lv
https://orcid.org/0000-0002-4042-0689
E-mail
roberts.matisons@silava.lv
- Purviņš, Latvian State Forest Research Institute Silava, 111 Rigas street, LV-2169, Salaspils, Latvia E-mail andis.purvins@silava.lv
- Augustovs, Latvian State Forest Research Institute Silava, 111 Rigas street, LV-2169, Salaspils, Latvia E-mail juris.augustovs@silava.lv
- Vīndedzis, Latvian State Forest Research Institute Silava, 111 Rigas street, LV-2169, Salaspils, Latvia E-mail valts.vindedzis@silava.lv
-
Jansons,
Latvian State Forest Research Institute Silava, 111 Rigas street, LV-2169, Salaspils, Latvia
 https://orcid.org/0000-0001-7981-4346
E-mail
aris.jansons@silava.lv
https://orcid.org/0000-0001-7981-4346
E-mail
aris.jansons@silava.lv
Received 8 February 2021 Accepted 30 April 2021 Published 11 May 2021
Views 57948
Available at https://doi.org/10.14214/sf.10524 | Download PDF

Supplementary Files
1 Introduction
Populations of tree species with wide geographic range, such as silver birch (Betula pendula Roth), show genetic specialization to native environments, while maintaining high phenotypical plasticity (Sultan 1987; Gapare et al. 2008). Silver birch has high genetic diversity, yet low population genetic differentiation (Hamrick et al. 1992; Palmé et al. 2003; Wesselink et al. 2018); e.g., 97% variation within the populations, and only up to 3% among them in the Eastern Baltic region (Zhuk et al. 2009). Nonetheless, explicit differentiation in phenotypic traits might be present due to strong local adaptation, and such genetic specialization is the precondition for successful breeding (Savolainen et al. 2007; Sork et al. 2013). In the Eastern Baltic region, silver birch is a highly important forest resource, especially for plywood industry (Hynynen et al. 2010; Liepins and Rieksts-Riekstins 2013). Accordingly, breeding programs are implemented throughout the region (Donaldson and Turner 2001; Heräjärvi 2001; Stener and Hedenberg 2003; Gailis et al. 2020).
Phenotypic traits have shown considerable genotypic variation in the Latvian silver birch breeding population (Gailis et al. 2020), yet the potential sub-regional differences have not been accounted. The species shows substantial ecological plasticity shaped by environmental contrasts (Koski and Rousi 2005; Savolainen et al. 2007), hence assessment of regional and sub-regional phenotypic differences (Falconer and Mackay 1996; Griffiths et al. 2000) is essential to improve efficiency of breeding (Malcolm and Worrell 2001). Bio-climatic zonation is commonly used to distinguish regional differences in tree growth, for example in classification of seed zones (Laiviņš and Melecis 2003; Hamann et al. 2011; Stakenas et al. 2012; Ge et al. 2013; Reitalu et al. 2013). However, the climatic zones might be different from the actual distribution of metapopulations of tree species. Different environmental conditions and the post-glacial history of vegetation interactively can result in spatially varying selection pressure, hence distinct phenotypic differences and genetic variance (Palmé et al. 2003; Väliranta et al. 2011; Tenkanen et al. 2020). Thus, assessment of the differences in phenotypes and specialization among forest provenances are still essential for clarification of provenance regions, which might not be detected by molecular markers (Karhu et al. 1996; Reed and Frankham 2001; O’brien et al. 2007; Wesselink et al. 2018).
Furthermore, the rapid pace of climate change rises concerns about the adaptative capacity of tree populations in the future (Aitken et al. 2008; Fady et al. 2020). Improved forest reproductive material enhances forest adaptation (Lefèvre et al. 2014) as a component of pro-active adaptive forest management (Bolte et al. 2009; Nabuurs et al. 2018). Considering the opportunistic (ruderal) nature of silver birch (Brzeziecki and Kienast 1994), fast growth and tolerance to weather fluctuation in the Eastern Baltic region (Liepiņš 2011; Jansons et al. 2016), a conservative climate-smart management approach may utilize improved local genotypes to enhance adaptability (Ahrens et al. 2020). Therefore, information about genetic variation and phenotypic plasticity reflecting adaptability of local seed sources (Lamy et al. 2011), is advantageous for more efficient breeding. The aim of the study was to distinguish sub-regional differences in strength of local adaptation in terms of growth and stem quality traits. We hypothesized that local bio-climate had an explicit effect on local adaptation, and two silver birch provenance regions could be delineated.
2 Material and methods
2.1 Trials and measurements
The study material consisted of the open-pollinated progenies of silver birch plus-trees from 31 forest provenances across Latvia (55°40´–58°05´N, 20°58´–28°14´E). The studied two parallel silver birch trials Taurene (57°06´N, 25°38´E) and Ukri (56°22´N, 23°07´E) contained progenies of the same sets of provenances and 533 half-sib families within them. The trials were established in 2000 on agricultural land with one-year-old containerized seedlings. The experimental design was complete randomized blocks of single-tree plots in 10 to 93 replications with 2 × 2.5 m initial spacing. Both trials were growing in mesotrophic conditions on dry silty soils. Climate was milder in the Ukri trial; the mean annual temperature was 6.4 °C, and the mean monthly temperature ranged from –4.4 °C to +17.4 °C in January and July, respectively; the mean annual precipitation was ca. 630 mm (Harris et al. 2020). In Taurene, the mean annual temperature was 5.1 °C, and the mean monthly temperature ranged from –6.3°C to +16.9 °C. The mean annual precipitation was ca. 670 mm (Harris et al. 2020).
At the age of 14 years, measurements of height (H) and diameter at breast height (DBH) were available for 11 657 and 18 804 trees in Taurene and Ukri trails, respectively. The occurrence of spike knots (SpKn), double leaders (Doubl), and lost top (LostTop) was recorded as binary variables. Stem straightness (StStr), overall stem quality (StQual), and branch angle (BrA) were assessed visually, using a 3-point ordinal scale (Gailis et al. 2020). Stem volume (StVol) was calculated according to a local equation (Liepa 1996).
2.2 Statistical analysis
We performed Principal Component Analysis to assess the main patterns in variation of scaled phenotypic traits of the studied silver birch provenances, and to associate them with the location of origin. Estimated marginal provenance means were obtained from mixed effects models:
![]()
, where yijk is the response variable, μ is the overall mean; Pi, Fj and Sk are the fixed effects of the provenance, the family, and the site, respectively. The bl and sfjk are the random effects of block and site × family interaction, respectively, and εijklm is the random residual effect. The significance of principal components (PC) was determined by the Monte Carlo (randomization) test performing 1000 iterations. Relationships of the studied traits and latitude/longitude with the first two PC were assessed by Pearson correlation analysis.
The phenotypic differences in the studied traits among the distinguished provenance regions were assessed using mixed models:
![]()
, where yijk is the response variable, Ri is the fixed effect of the provenance region, sj is the fixed effect of site, bk is the random effect of a block within a site, and εijk is the error.
To estimate the extent of genetic adaptation and genetically determined plasticity for each of the determined provenance regions, the variance components were estimated from the combined data from both trials, and narrow-sense heritability (h2) and additive genetic coefficient of variation (CVa) were calculated according to Falconer and Mackay (1996).
For the continuous quantitative variables (H, DBH, StVol), linear mixed effects models were used. For the binomial variables (SpKn, Doubl, LostTop), generalised linear mixed models applying binomial residual distribution and a “logit” link function were fitted. For the ordinal variables (StStr, StQual, BrA), ordinal logistic regression was applied (Long 1997). Data analysis was conducted in SAS v. 9.3 using the procedures PROC MIXED, PROC GLIMMIX, and PROC CORR (Littel et al. 2006; Piepho and Möhring 2011).
3 Results
Distinct clustering of phenotypic traits was observed. The first two PCs were significant (p-value < 0.001) and corresponded for 57.6% of the total variance of the studied traits (Fig. 1). The third PC covered 19.8% of the variance and was related to stem defects – Lost and Doubl. The first PC was strongly related to the growth traits indicating regional differences in productivity (Fig. 1). The second PC was related to the quality traits, suggesting diverse sources of variation for growth and quality. In the ordination space, the provenances formed single group indicating continuous gradient in variation of the studied traits. Nevertheless, the first PC was significantly correlated with the longitude of origin of the provenances (r = 0.46, p = 0.01), suggesting growth differences between the coastal and inland parts of the country, while the second PC did not (r = 0.02, p = 0.92).
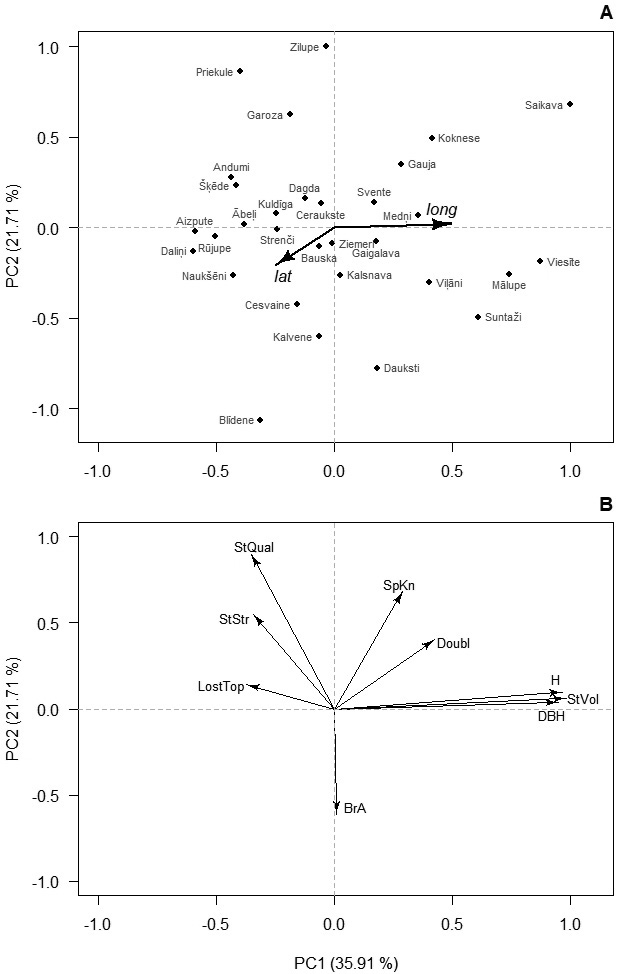
Fig. 1. Ordination of the studied provenances (A) and studied traits (B) of 14-years-old silver birch in Latvia according to the first two principal components (PC) of their variation. In A, axes are rescaled for clarity; and arrows indicate correlation with latitude (lat) and longitude (lon) of origin of the provenances. Numbers in brackets indicate the amount of explained variation. Abbreviations: H – height, DBH – diameter at breast height, StVol – stem volume, Doubl –probability of double leaders, SpKn – spike knots, StQual – overall stem quality, StStr – stem straightness, LostTop – lost top, BrA – branch angle.
Correlations of the PCs with latitude of origin were weak (|r| < 0.22, p-value > 0.24), indicating absence of north-south gradient. Based on the scores of the first PC of provenances, which were overlain on their geographic locations, two regions of Latvia were arbitrarily distinguished (Fig. 2). The coastal region included the western and the very northern part of the country, while the inland region covered the central and eastern parts (Fig. 2). The inland region showed significantly (p-value < 0.01) higher H, DBH, and StVol (Table 1). The mean StStr, StQual, and BrA had no differences between regions. High occurrence of SpKn and LostTop was observed in both regions (49.2–59.1%), while trees with Doubl were less frequent, lacking practically meaningful differences between regions (Table 1).
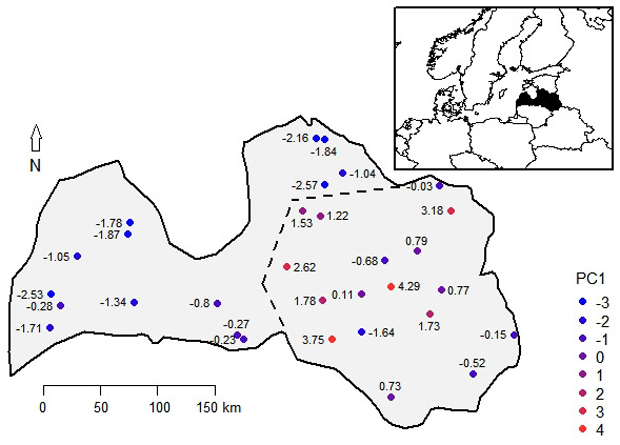
Fig. 2. The scores of the first principal component (PC1) of studied phenotypic traits of 14-years-old silver birch progenies overlain on their geographic locations in Latvia. The dotted line indicates border of two arbitrarily distinguished silver birch provenance regions – the coastal (western) and the inland (eastern) region.
| Table 1. Regional means, minimum and maximum provenance means, narrow sense individual tree heritability and additive genetic coefficient of variation of the studied traits in the phenotypically distinguished provenance regions of silver birch in Latvia. Letters in uppercase denote significant differences (p ≤ 0.05) between provenance regions for each trait. | ||||||||
| Trait | Regional mean ± standard deviation | Minimum provenance mean | Maximum provenance mean | Individual tree heritability h2 ± standard error (additive genetic coefficient of variation CVa, %) | ||||
| Coastal | Inland | Coastal | Inland | Coastal | Inland | Coastal | Inland | |
| Height (m) | 11.8 A ± 1.90 | 12.3 B ± 1.89 | 11.2 | 11.7 | 12.0 | 12.5 | 0.28 ± 0.037 (7.16) | 0.61 ± 0.061 (10.11) |
| Diameter at breast height (cm) | 9.06 A ± 2.81 | 9.8 B ± 2.84 | 8.5 | 8.7 | 10.0 | 10.7 | 0.32 ± 0.037 (16.56) | 0.42 ± 0.048 (17.53) |
| Stem volume (dm3) | 45.2 A ± 26.56 | 53.2 B ± 29.87 | 39.3 | 43.2 | 49.2 | 62.1 | 0.29 ± 0.035 (29.35) | 0.41 ± 0.048 (33.41) |
| Spike knots (% of trees) | 57.3 A | 59.1 B | 42.1 | 53.6 | 60.8 | 65.4 | 0.05 ± 0.017 | 0.07 ± 0.020 |
| Double leaders (% of trees) | 9.7 A | 11.4 B | 2.8 | 5.00 | 10.4 | 13.9 | 0.15 ± 0.041 | 0.16 ± 0.014 |
| Lost top (% of trees) | 52.3 A | 49.2 B | 50.7 | 46.2 | 65.6 | 62.4 | 0.03 ± 0.017 | 0.01 ± 0.017 |
| Stem straightness score | 2.2 A | 2.2 A | 2.1 | 2.1 | 2.2 | 2.2 | 0.45 ± 0.060 | 0.26 ± 0.060 |
| Overall stem quality score | 2.8 A | 2.8 A | 2.7 | 2.7 | 2.8 | 2.9 | 0.24 ± 0.040 | 0.28 ± 0.061 |
| Branch angle score | 2.0 A | 2.0 A | 2.0 | 2.0 | 2.0 | 2.0 | 0.51 ± 0.110 | 0.30 ± 0.118 |
The studied silver birch populations possessed substantial additive genetic variance in growth and stem quality (Supplementary file S1). Moderate to high heritability (h2 > 0.20) was estimated for the studied traits in both regions except for stem defects (h2 < 0.15) (Table 1). Geographically varying strength of genetic control of the traits was observed: h2 of H was more than twice higher in the inland than in the coastal region (0.61 ± 0.061 and 0.28 ± 0.037, respectively), while for StVol and DBH differences in h2 reached 31.3–41.4%. In the coastal region, high (h2 ≥ 0.45) heritability was estimated for StStr and BrA, while these traits showed intermediate heritability (0.26 ≤ h2 ≤ 0.30) in the inland. Also, the estimated CVa was slightly (0.97–4.06%) higher for the inland comparing to the coastal region implying slight differences in plasticity. The ability to respond to natural selection, indicated by CVa, was ca. three times higher for StVol comparing to H in both regions (Table 1).
4 Discussion
Phenotypic plasticity and local (genetic) specialization are key factors affecting adaptability of trees to changing climate, hence crucial for climate-smart forest management (Aitken and Bemmels 2016; Moran et al. 2017). Distinct local specialization of silver birch was observed, particularly for the growth traits (Table 1). Silver birch of the inland region possessed better growth (Table 1, Fig. 1). The explicit coastal-inland gradient (Fig. 1) and differing strength of genetic control (Table 1) followed general spatial pattern of the climatic zonation within the region (Laiviņš and Melecis 2003; Reitalu et al. 2013).
The distribution of silver birch provenance regions showed some specifics in growth traits and their genotypic variation (Fig. 2, Table 1), which might be explained by the post-glacial recolonization routes from different refugees (Palmé et al. 2003; Kapeller et al. 2017; Tsuda et al. 2017; Tenkanen et al. 2020). Also, this might be due to continentality of climate (White et al. 2007; Hoffmann and Sgrò 2011), as determined by westerlies and proximity of the Baltic Sea (Laiviņš and Melecis 2003); hence differing strength of environmental forcing of adaptation (Suppl. file S2).
Population genetic studies have shown persistence of silver birch in relatively high latitudes during last glacial maximum, as a scattered dispersal nuclei enabling rapid post-glacial recolonization in different directions (Stewart and Lister 2001; Binney et al. 2009; Väliranta et al. 2011; Kapeller et al. 2017; Tsuda et al. 2017). The inland region appeared to be westwards extension of a source population located east from Latvia. Still, such dispersal nuclei are difficult to locate (Amon et al. 2014), and wider regional scale study would be necessary to clarify this issue. However, natural selection after the post-glacial recolonization has likely been the main driver determining geographic variation of studied traits (Fig. 2) as observed in the region (Collignon et al. 2002; Kremer et al. 2002; Savolainen et al. 2007; Väliranta et al. 2011).
Although high within- and low between-population genetic diversity has been observed for silver birch in Northern and Eastern Europe (Hamrick et al. 1992; Palmé et al. 2003; Rusanen et al. 2003; Maliouchenko et al. 2007; Zhuk et al. 2009), the former has apparently favoured strong specialization to local conditions, hence explicit phenotypical differences (White et al. 2007; Hoffmann and Sgrò 2011). Considerably higher CVa for StVol (29.35–33.41%) comparing to H and DBH (7.16–17.43%) corresponded to a common trend in forest trees (Cornelius 1994). The heritability is affected by the history of the region (Falconer and Mackay 1996; Griffiths et al. 2000), and can vary greatly for phenotypic traits of forest tree species (Cornelius 1994). Higher genetic specialization was more evident under more continental climate in the inland region (Fig. 2), resulting in higher heterogeneity of field performance (Fig. 1), and explained higher h2 and CVa of growth traits, compared to the coastal region (Table 1). High heritability likely indicated larger differences between genotypes from different forest provenances comparing to the environmental variation within genotypes (Griffiths et al. 2000). Estimated h2 for growth traits was higher in the inland and lower in the coastal provenances comparing to the whole breeding population (h2 = 0.41–0.52) (Gailis et al. 2020), indicating better response to selection in the first. One commonly acknowledged risk of intensive breeding within certain populations can be reduced genetic diversity, yet threats to silver birch are unlikely, considering the highly-connected populations with extensive gene flow (Hoban and Schlarbaum 2014).
Indistinct phenotypic differences and similar moderate to high heritability for stem quality (Table 1) might have been set by uniform natural selection in both regions despite climatic differences (Lamy et al. 2011). Still, moderate genetic control of StStr, previously reported for various trees species (Cornelius 1994), indicated potential improvement of selection. Meanwhile weak genetic control of stem defects (Table 1) correspond to the earlier findings, likely shaped by prevailing environmental factors, such as frost, insect damage or browsing (Malcolm and Worrell 2001; Stener and Jansson 2005; Zeltiņš et al. 2018).
Heritability and CVa reflect pre-existing standing genetic variation, which is essential to adapt to a broad spectrum of future climate via natural selection (Alberto et al. 2013; Ahrens et al. 2020). Although low genetic variance not necessarily means poor selection response (Walsh and Blows 2009; Hoffmann and Sgrò 2011), lower h2 of growth traits in coastal region (Table 1) could indicate weaker adaptability to ongoing climate change (Hoffmann and Sgrò 2011). Still, it is unclear, whether populations currently possessing weaker local adaptation could show better fitness in the future (Fady et al. 2020). Coastal provenances could benefit from projected milder winters and increased precipitation intensity thorough the country, yet more frequent extreme events (e.g. summer drought) may suppress positive effect (Avotniece et al. 2010). Use of robust superior seed sources from more continental inland climate may imply potentially higher capacity to adapt to changing conditions (Sork et al. 2013), facilitating resilience of future stands (Aitken and Bemmels 2016). Still, the indistinct phenotypical and genetic variation for stem quality traits suggested potential for improvement of breeding for plywood production also combining material, with higher preference from the inland region.
5 Conclusions
Delineation of two provenance regions – coastal and inland – for silver birch in Latvia with respect to growth performance and genotypic variation, justified earlier climatic zonation. Overall, better growth and higher heritability suggests that selection and breeding within the best provenances in more continental inland region possessing higher genotypic variation may constitute better performing and adaptable breeding population for climate-smart management comparing to the coastal region. Still, uniformity and estimated heritability of quality traits implies improved stemwood quality for plywood production regardless of the distinguished region. A wider regional scale study, however, would be necessary to clarify differences in phenotypic and genotypic variation as a proxy for adaptation capacity to changing climate.
Declaration of openness of research materials, data, and code
The data that support the findings of this study are available from the corresponding author, PZ, upon reasonable request.
Authors’ contributions
AG and ĀJ developed design of the work, conceptualized research question, and revised the work. PZ and RM conducted data analysis and revised the work. AP, JA and VV were responsible for data acquisition. All authors contributed to scientific writing of the work.
Funding
The study was funded by European Regional Development fund project (No. 1.1.1.1/19/A/111) “Decision support tool for increased forest productivity via efficient climate adjusted transfer of genetic gain”.
References
Ahrens CW, Andrew ME, Mazanec RA, Ruthrof KX, Challis A, Hardy G, Byrne M, Tissue DT, Rymer PD (2020) Plant functional traits differ in adaptability and are predicted to be differentially affected by climate change. Ecol Evol 10: 232–248. https://doi.org/10.1002/ece3.5890.
Aitken SN, Bemmels JB (2016) Time to get moving: assisted gene flow of forest trees. Evol Appl 9: 271–290. https://doi.org/10.1111/eva.12293.
Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S (2008) Adaptation, migration or extirpation: climate change outcomes for tree populations. Evol Appl 1: 95–111. https://doi.org/10.1111/j.1752-4571.2007.00013.x.
Alberto FJ, Derory J, Boury C, Frigerio JM, Zimmermann NE, Kremer A (2013) Imprints of natural selection along environmental gradients in phenology-related genes of Quercus petraea. Genetics 195: 495–512. https://doi.org/10.1534/genetics.113.153783.
Amon L, Veski S, Vassiljev J (2014) Tree taxa immigration to the eastern Baltic region, southeastern sector of Scandinavian glaciation during the Late-glacial period (14,500–11,700 cal. b.p.). Veg Hist Archaeobot 23: 207–216. https://doi.org/10.1007/s00334-014-0442-6.
Avotniece Z, Rodinov V, Lizuma L, Briede A, Kļaviņš M (2010) Trends in the frequency of extreme climate events in Latvia. Baltica 23:135–148.
Binney HA, Willis KJ, Edwards ME, Bhagwat SA, Anderson PM, Andreev AA, Blaauw M, Damblon F, Haesaerts P, Kienast F, Kremenetski KV, Krivonogov SK, Lozhkin AV, MacDonald GM, Novenko EY, Oksanen P, Sapelko TV, Väliranta M, Vazhenina L (2009) The distribution of late-Quaternary woody taxa in northern Eurasia: evidence from a new macrofossil database. Quat Sci Rev 28: 2445–2464. https://doi.org/10.1016/J.QUASCIREV.2009.04.016.
Bolte A, Ammer C, Löf M, Madsen P, Nabuurs GJ, Schall P, Spathelf P, Rock J (2009) Adaptive forest management in central Europe: climate change impacts, strategies and integrative concept. Scand J For Res 24: 473–482. https://doi.org/10.1080/02827580903418224.
Brzeziecki B, Kienast F (1994) Classifying the life-history strategies of trees on the basis of the Grimian model. For Ecol Manage 69: 167–187. https://doi.org/10.1016/0378-1127(94)90227-5.
Collignon A-M, van de Sype H, Favre J-M (2002) Geographical variation in random amplified polymorphic DNA and quantitative traits in Norway spruce. Can J For Res 32: 266–282. https://doi.org/10.1139/x01-198.
Cornelius J (1994) Heritabilities and additive genetic coefficients of variation in forest trees. Can J For Res 24: 372–379. https://doi.org/10.1139/x94-050.
Donaldson LA, Turner JCP (2001) The influence of compression wood and microfibril angle on the occurrence of distortion in window frames made from radiata pine (Pinus radiata). Holz als Roh- und Werkst 59: 163–168. https://doi.org/10.1007/s001070100201.
Fady B, Aravanopoulos F, Benavides R, González-Martínez S, Grivet D, Lascoux M, Lindner M, Rellstab C, Valladares F, Vinceti B (2020) Genetics to the rescue: managing forests sustainably in a changing world. Tree Genet Genomes 16, article id 80. https://doi.org/10.1007/s11295-020-01474-8.
Falconer DS, Mackay TF (1996) Introduction to quantitative genetics 4th edition. Longman Group Ltd, London.
Gailis A, Zeltiņš P, Purviņš A, Augustovs J, Vīndedzis V, Zariņa I, Jansons Ā (2020) Genetic parameters of growth and quality traits in open-pollinated silver birch progeny tests. Silva Fenn. 54, article id 10220. https://doi.org/10.14214/sf.10220.
Gapare WJ, Yanchuk AD, Aitken SN (2008) Optimal sampling strategies for capture of genetic diversity differ between core and peripheral populations of Picea sitchensis (Bong.) Carr. Conserv Genet 9: 411–418. https://doi.org/10.1007/s10592-007-9353-8.
Ge ZM, Kellomäki S, Peltola H, Zhou X, Väisänen H, Strandman H (2013) Impacts of climate change on primary production and carbon sequestration of boreal Norway spruce forests: Finland as a model. Clim Change 118: 259–273. https://doi.org/10.1007/s10584-012-0607-1.
Griffiths A, Miller J, Suzuki D, Lewontin RC, Gelbart WM (2000) Quantifying heritability. In: Griffiths A, Miller J, Suzuki D, Lewontin RC, Gelbart WM (eds) An introduction to genetic analysis. WH Freeman, New York.
Hamann A, Gylander T, Chen P (2011) Developing seed zones and transfer guidelines with multivariate regression trees. Tree Genet Genomes 7: 399–408. https://doi.org/10.1007/s11295-010-0341-7.
Hamrick JL, Godt MJW, Sherman-Broyles SL (1992) Factors influencing levels of genetic diversity in woody plant species. Springer, Dordrecht, pp 95–124. https://doi.org/10.1007/978-94-011-2815-5_7.
Harris I, Osborn TJ, Jones P, Lister D (2020) Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci Data 7: 1–18. https://doi.org/10.1038/s41597-020-0453-3.
Heräjärvi H (2001) Technical properties of mature birch (Betula pendula and B. pubescens) for saw milling in Finland. Silva Fenn 35: 469–485. https://doi.org/10.14214/sf.581.
Hoban S, Schlarbaum S (2014) Optimal sampling of seeds from plant populations for ex-situ conservation of genetic biodiversity, considering realistic population structure. Biol Conserv 177: 90–99. https://doi.org/10.1016/j.biocon.2014.06.014.
Hoffmann AA, Sgrò CM (2011) Climate change and evolutionary adaptation. Nature 470: 479–485. https://doi.org/10.1038/nature09670.
Hynynen J, Niemisto P, Vihera-Aarnio A, Brunner A, Hein S, Velling P (2010) Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in northern Europe. Forestry 83: 103–119. https://doi.org/10.1093/forestry/cpp035.
Jansons Ā, Matisons R, Šēnhofa S, Katrevičs J, Jansons J (2016) High-frequency variation of tree-ring width of some native and alien tree species in Latvia during the period 1965–2009. Dendrochronologia 40: 151–158. https://doi.org/10.1016/j.dendro.2016.10.003.
Kapeller S, Dieckmann U, Schueler S (2017) Varying selection differential throughout the climatic range of Norway spruce in Central Europe. Evol Appl 10: 25–38. https://doi.org/10.1111/eva.12413.
Karhu A, Hurme P, Karjalainen M, Karvonen P, Kärkkäinen K, Neale D, Savolainen O (1996) Do molecular markers reflect patterns of differentiation in adaptive traits of conifers? Theor Appl Genet 93: 215–221. https://doi.org/10.1007/BF00225748.
Koski V, Rousi M (2005) A review of the promises and constraints of breeding silver birch (Betula pendula Roth) in Finland. For An Int J For Res 78: 187–198. https://doi.org/10.1093/forestry/cpi017.
Kremer A, Kleinschmit J, Cottrell J, Cundall EP, Deans JD, Ducousso A, König AO, Lowe AJ, Munro RC, Petit RJ, Stephan BR (2002) Is there a correlation between chloroplastic and nuclear divergence, or what are the roles of history and selection on genetic diversity in European oaks? For Ecol Manage 156: 75–87. https://doi.org/10.1016/S0378-1127(01)00635-1.
Laiviņš M, Melecis V (2003) Bio-geographical interpretation of climate data in Latvia: multidimensional analysis. Acta Univ Latv 654: 7–22.
Lamy J-B, Bouffier L, Burlett R, Plomion C, Cochard H, Delzon S (2011) Uniform selection as a primary force reducing population genetic differentiation of cavitation resistance across a species range. PLoS One 6, article id e23476. https://doi.org/10.1371/journal.pone.0023476.
Lefèvre F, Boivin T, Bontemps A, Courbet F, Davi H, Durand-Gillmann M, Fady B, Gauzere J, Gidoin C, Karam MJ, Lalagüe H, Oddou-Muratorio S, Pichot C (2014) Considering evolutionary processes in adaptive forestry. Ann For Sci 71: 723–739. https://doi.org/10.1007/s13595-013-0272-1.
Liepa I (1996) Pieauguma mācība [Increment science]. LLU, Jelgava.
Liepiņš K (2011) Growth of silver birch (Betula pendula Roth) in plantations on farmlands in Latvia. Mežzinātne 23: 3–14.
Liepins K., Rieksts-Riekstins J (2013) Stemwood density of juvenile silver birch trees (Betula pendula Roth) from plantations on former farmlands. Balt For 19: 179–186.
Littel RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models 2nd edition. SAS Publishing, Cary, NC.
Malcolm DC, Worrell R (2001) Potential for the improvement of silver birch (Betula pendula Roth.) in Scotland. Forestry 74: 439–453. https://doi.org/10.1093/forestry/74.5.439.
Maliouchenko O, Palmé AE, Buonamici A, Vendramin GG, Lascoux M (2007) Comparative phylogeography and population structure of European Betula species, with particular focus on B. pendula and B. pubescens. J Biogeogr 34: 1601–1610. https://doi.org/10.1111/j.1365-2699.2007.01729.x.
Moran E, Lauder J, Musser C, Stathos A, Shu M (2017) The genetics of drought tolerance in conifers. New Phytol 216: 1034–1048. https://doi.org/10.1111/nph.14774.
Nabuurs G-J, Verkerk PJ, Schelhaas M, González-Olabarria JR, Trasobares A, Cienciala E (2018) Climate-smart forestry: mitigation implact in three European regions. From Science to Policy 6. European Forest Institute, Joensuu.
O’Brien EK, Mazanec RA, Krauss SL (2007) Provenance variation of ecologically important traits of forest trees: implications for restoration. J Appl Ecol 44: 583–593. https://doi.org/10.1111/j.1365-2664.2007.01313.x.
Palmé AE, Su Q, Rautenberg A, Manni F, Lascoux M (2003) Postglacial recolonization and cpDNA variation of silver birch, Betula pendula. Mol Ecol 12: 201–212. https://doi.org/10.1046/j.1365-294X.2003.01724.x.
Piepho HP, Möhring J (2011) On estimation of genotypic correlations and their standard errors by multivariate REML using the MIXED procedure of the SAS System. Crop Sci 51: 2449–2454. https://doi.org/10.2135/cropsci2011.02.0088.
Reed DH, Frankham R (2001) How closely correlated are molecular and quantitative measures of genetic variation? A meta-analysis. Evolution 55: 1095–1103. https://doi.org/10.1111/j.0014-3820.2001.tb00629.x.
Reitalu T, Seppä H, Sugita S, Kangur M, Koff T, Avel E, Kihno K, Vassiljev J, Renssen H, Hammarlund D, Heikkilä M, Saarse L, Poska A, Veski S (2013) Long-term drivers of forest composition in a boreonemoral region: the relative importance of climate and human impact. J Biogeogr 40: 1524–1534. https://doi.org/10.1111/jbi.12092.
Rusanen M, Vakkari P, Blom A (2003) Genetic structure of Acer platanoides and Betula pendula in northern Europe. Can J For Res 33: 1110–1115. https://doi.org/10.1139/x03-025.
Savolainen O, Pyhäjärvi T, Knürr T (2007) Gene flow and local adaptation in trees. Annu Rev Ecol Evol Syst 38: 595–619. https://doi.org/10.1146/annurev.ecolsys.38.091206.095646.
Sork VL, Aitken SN, Dyer RJ, Eckert AJ, Legendre P, Neale DB (2013) Putting the landscape into the genomics of trees: approaches for understanding local adaptation and population responses to changing climate. Tree Genet 9: 901–911. https://doi.org/10.1007/s11295-013-0596-x.
Stakenas V, Žemaitis P, Ozolinčius R (2012) Crown condition of Norway spruce in different eco-climatic regions of Lithuania: implications for future climate. Balt For 18: 187–195.
Stener L-G, Jansson G (2005) Improvement of Betula pendula by clonal and progeny testing of phenotypically selected trees. Scand J For Res 20: 292–303. https://doi.org/10.1080/02827580510036265.
Stener L-G, Hedenberg Ö (2003) Genetic parameters of wood, fibre, stem quality and growth traits in a clone test with Betula pendula. Scand J For Res 18: 103–110. https://doi.org/10.1080/02827580310003678.
Stewart JR, Lister AM (2001) Cryptic northern refugia and the origins of the modern biota. Trends Ecol Evol 16: 608–613. https://doi.org/10.1016/S0169-5347(01)02338-2.
Sultan SE (1987) Evolutionary implications of phenotypic plasticity in plants. Evol Biol 21: 127–178. https://doi.org/10.1007/978-1-4615-6986-2_7.
Tenkanen A, Keski-Saari S, Salojärvi J, Oksanen E, Keinänen M, Kontunen-Soppela S (2020) Differences in growth and gas exchange between southern and northern provenances of silver birch (Betula pendula Roth) in northern Europe. Tree Physiol 40: 198–214. https://doi.org/10.1093/treephys/tpz124.
Tsuda Y, Semerikov V, Sebastiani F, Vendramin GG, Lascoux M (2017) Multispecies genetic structure and hybridization in the Betula genus across Eurasia. Mol Ecol 26: 589–605. https://doi.org/10.1111/mec.13885.
Väliranta M, Kaakinen A, Kuhry P, Kultti S, Salonen JS, Seppä H (2011) Scattered late-glacial and early Holocene tree populations as dispersal nuclei for forest development in north-eastern European Russia. J Biogeogr 38: 922–932. https://doi.org/10.1111/j.1365-2699.2010.02448.x.
Walsh B, Blows MW (2009) Abundant genetic variation + strong selection = multivariate genetic constraints: a geometric view of adaptation. Annu Rev Ecol Evol Syst 40: 41–59. https://doi.org/10.1146/annurev.ecolsys.110308.120232.
Wesselink M, Dragutinović A, Noordhoek JW, Bergwerff L, Kuiper I (2018) DNA typing of birch: development of a forensic STR system for Betula pendula and Betula pubescens. Forensic Sci Int Genet 35: 70–81. https://doi.org/10.1016/j.fsigen.2018.04.001.
White TL, Adams WT, Neale DB (2007) Forest genetics. CABI Publishing, UK. https://doi.org/10.1038/167764a0.
Zeltiņš P, Matisons R, Gailis A, Jansons J, Katrevičs J, Jansons Ā (2018) Genetic parameters of growth traits and stem quality of silver birch in a low-density clonal plantation. Forests 9, article id 52. https://doi.org/10.3390/f9020052.
Zhuk A, Šķipars V, Veinberga I, Gailis A, Ruņģis D (2009) Assessment of genetic diversity in Latvian silver birch Betula pendula Roth populations. Latv Veģetācija 18: 5–12.
Total of 62 references.

