Forest fires have long-term effects on the composition of vascular plants and bryophytes in Scots pine forests of hemiboreal Estonia
Orumaa A., Köster K., Tullus A., Tullus T., Metslaid M. (2022). Forest fires have long-term effects on the composition of vascular plants and bryophytes in Scots pine forests of hemiboreal Estonia. Silva Fennica vol. 56 no. 1 article id 10598. https://doi.org/10.14214/sf.10598
Highlights
- We recorded 31 vascular plant and 39 bryophyte species in a chronosequence of Scots pine stands with 12–183 years since fire
- Time since fire affected the compositional patterns of vascular plants and bryophytes
- The richness of liverworts was higher in recently burned stands due to the presence of Cephaloziella spp.
- The richness of dwarf-shrubs increased with longer period since fire.
Abstract
Since fire frequency is expected to increase globally due to climate change, it is important to understand its effects on forest ecosystems. We studied the long-term patterns in species diversity, cover and composition of vascular plants and bryophytes after forest fire and the site-related factors behind them. Research was carried out in northwestern Estonia, using a chronosequence of Scots pine (Pinus sylvestris L.) stands, located on nutrient poor sandy soils, where fires had occurred 12, 23, 38, 69, 80 and 183 years ago. In every stand three 100 m2 vegetation plots were established to collect floristic and environmental information. The effects on floristic characteristics of time since fire, light, and soil variables were evaluated with linear mixed models, followed by backward variable selection. Compositional variation was analysed with non-metric multidimensional scaling, Multi-response Permutation Procedures, and Indicator Species Analysis. Altogether, 31 vascular plant and 39 bryophyte species were found in vegetation plots. The cover of the vascular plant and bryophyte layers increased with a longer time since fire. Soil and light variables impacted the richness of several vascular plant and bryophyte groups, whereas only the richness of liverworts and dwarf-shrubs correlated with time since fire. Considerable compositional differences were observed in vascular plant and bryophyte assemblages between recently vs. long-time ago burned stands. To conclude, time since fire significantly impacted compositional patterns of vascular plants and bryophytes in pine forests on nutrient poor soils, although time-related trends in species richness were less evident.
Keywords
disturbance;
bryophytes;
understorey vegetation;
vascular plants;
hemiboreal forest;
fire chronosequence;
wildfire
-
Orumaa,
Institute of Forestry and Engineering, Estonian University of Life Sciences, Kreutzwaldi 5, 51006, Tartu, Estonia
E-mail
argo.orumaa@emu.ee

- Köster, Department of Environmental and Biological Sciences, University of Eastern Finland, PO Box 111 (Yliopistokatu 7), 80130, Joensuu, Finland E-mail kajar.koster@helsinki.fi
- Tullus, Department of Botany, Institute of Ecology and Earth Sciences, University of Tartu, Lai 40, Tartu, 51003, Estonia E-mail arvo.tullus@ut.ee
- Tullus, Institute of Forestry and Engineering, Estonian University of Life Sciences, Kreutzwaldi 5, 51006, Tartu, Estonia E-mail tea.tullus@emu.ee
- Metslaid, Institute of Forestry and Engineering, Estonian University of Life Sciences, Kreutzwaldi 5, 51006, Tartu, Estonia E-mail marek.metslaid@emu.ee
Received 17 June 2021 Accepted 18 January 2022 Published 31 January 2022
Views 73275
Available at https://doi.org/10.14214/sf.10598 | Download PDF

Supplementary Files
1 Introduction
Fire is one of the main disturbances in the hemiboreal forest zone (Jõgiste et al. 2017). It has a huge impact on forest ecosystems, altering forest structure and species composition (Franklin et al. 2002), soil properties and nutrient pools (Köster et al. 2016), understorey vegetation (Marozas et al. 2007; Parro et al. 2009), and forest fauna (Úbeda and Sarricolea 2016). Due to climate change, increased average air temperatures and more frequent and longer periods of droughts are expected, thereby affecting fire occurrence time (seasonal variation), frequency (shorter fire return interval), intensity (the released energy from the fire), severity (the mortality of trees, canopy loss, etc.) and the size of area burned (Flannigan et al. 2009; Seidl et al. 2011; Walker et al. 2019).
Understorey vegetation is less protected against fire than trees due to the lack of protective structures such as thick bark. Tree species such as Scots pine (Pinus sylvestris L.) can survive low to moderate severity fires, thanks to thick bark at older ages (Kuuluvainen et al. 2002), while understorey vegetation and smaller trees are killed. Survival of understorey vegetation depends on fire severity, as high intensity fires can remove the litter and soil humus layers, causing loss of plant roots, seeds, root corms and rhizomes (Schimmel and Granstrom 1996). The responses of different taxonomic groups to fires with different intensity are highly variable and the time needed for postfire recovery of species diversity, composition, and abundance may range from short (a few years) to long (>100 years) time intervals (Gorshkov and Bakkal 1996; Marozas et al. 2007; Bartels and Chen 2015).
Fires trigger successional changes in understorey vascular plant and bryophyte communities (Jean et al. 2017; Liu et al. 2020) as wildfire provides more opportunities for new species to colonize a site in the exposed gap areas with more light and exposed mineral soil (Marozas et al. 2007; Parro et al. 2009). Burned areas have higher pH, thinner soil humus layers, and increased available nutrients (Simard et al. 2001; Certini 2005), making an environment suitable for plants to grow. Nevertheless, as time since fire proceeds, soil nutrient availability and pH decrease (Brais et al. 1995; Liu et al. 2017; Paré and Bergeron 1996).
Fire-altered environmental conditions often lead to higher diversity and abundance of understorey vegetation in boreal forests due to the inflow of disturbance-adapted and rapidly growing species that benefit from improved light conditions and increased amount of nutrients (Grandpré et al. 1993; Marozas et al. 2007). Throughout post-fire succession, decreases in nutrient and light availability bring changes in species diversity and abundance, although the direct effects on floristic characteristics may vary, depending on the taxonomic (e.g., vascular plants vs. bryophytes) (Hart and Chen 2008) or functional group (e.g., shrubs vs. herbs, mosses vs. liverworts) (Paquette et al. 2016; Liu et al. 2020). Different plant functional groups are known to dominate different stages of post-fire succession in boreal forests, e.g., pioneer species (both vascular plants as well as bryophytes) with high dispersal rates become dominant shortly after fire and decline gradually over the next decades, whereas late successional species (e.g., pleurocarpous feather-mosses) increase in abundance in later successional stages (Grandpré et al. 1993; Marozas et al. 2007; Paquette et al. 2016; Jean et al. 2017, 2019). As many bryophyte species have specific substrate requirements (soil, dead-wood, trunks and bases of different tree species) (Mills and Macdonald 2005), their recovery in post-fire stands should depend on the availability of suitable substrates. However, the dynamics of bryophyte communities on different substrate types after wildfires have rarely been studied (Paquette et al. 2016; Kumar et al. 2017, 2018a) and the majority of studies addressing bryophytes have concentrated on ground-dwelling species.
As the impacts of wildfires on forest biodiversity may be long-term (Kuuluvainen 2002), the chronosequence method, consisting of post-fire stands with variable time since fire, has frequently been used to study the recovery patterns of vascular plants and bryophytes in the boreal forests of North America (Grandpré et al. 1993; Hart and Chen 2008; Paquette et al. 2016; Jean et al. 2017, 2019). In northern Europe the method seldomly has been applied for studying the long-term impacts of fire on understorey vegetation (Parro et al. 2009; Gorshkov and Bakkal 1996), despite the significant history of forest fires in the area (Donis et al. 2017). Although the space-for-time substitution (i.e., chronosequence) has been criticized because the assumption of stability over the time span of successional sequences is considered unlikely (Johnson and Miyanishi 2008), the method has been found to be appropriate for studying communities with low biodiversity and following convergent successional trajectories (Walker et al. 2010). The post-fire recovery of pine stands in nutrient poor habitats meets these assumptions.
In this study, we analysed the patterns of vascular plant and bryophyte vegetation in a post-fire chronosequence of Scots pine stands, ranging from 12 to 183 years since the last fire and growing on nutrient poor sandy soils. The main aim was to study the post-fire dynamics of species diversity, cover, and composition of vascular plants and bryophytes, and to identify the site-related factors that determined changes in different post-fire patterns. We expected to see significant differences in vascular plant and bryophyte species composition between recently burned vs. stands burned a long-time ago, but less clear trends for overall species richness throughout the post-fire succession, as recently burned stands are expected to host more early successional species compared to stands burned a long time ago. In addition, we sought to clarify the impact of time since fire on the richness of different species groups (shrubs and young trees, dwarf-shrubs, graminoids, forbs, liverworts and mosses). Separate analyses were conducted for bryophytes recorded on different substrate types.
2 Materials and methods
2.1 Study area
The study area is located in northwestern Estonia (Fig. 1), in Nõva and Vihterpalu area, situated within 145 km2. According to the closest weather station of the Estonian Weather Service in Lääne-Nigula (approximately 30 km from sample plots, 58°57´N, 23°48´E) the mean long-term (1991–2020) temperature is +6.4 °C and average annual precipitation is 688 mm. The coldest month is February, at –3.6 °C and the warmest is July, at +17.6 °C while average precipitation is lowest in April (36 mm) and highest in September (80 mm) (Estonian Weather Service 2021).
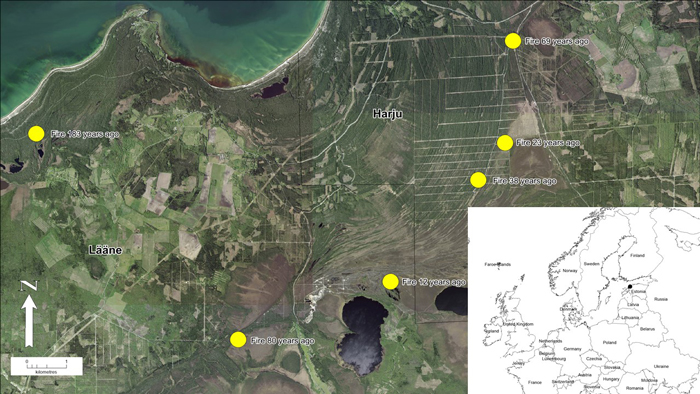
Fig 1. Location of study area in northwestern Estonia. Yellow dots represent pine stands (n = 6) with different age (time since fire) in the post-fire chronosequence. View larger in new window/tab.
Six areas were chosen for the post-fire chronosequence (Köster et al. 2016), where fires took place in 1837, 1940, 1951, 1982, 1997 and 2008 (Table 1). All wildfires were either completely stand-replacing or most of the stands were destroyed by fire. The area burned by wildfires ranged from 200 ha to more than 2000 ha. The age of stands at the time of fires varied between 30 to 70 years or is unknown. The information about stands before fire was taken from old forest inventory data. Unfortunately, for some areas data was unavailable (stands burned 80 and 183 years ago). All stands were located on sandy soils and were similar in terms of microclimate and elevation (Köster et al. 2016; Ribeiro-Kumara et al. 2019).
| Table 1. Main characteristics of studied stands (n = 6) in the post-fire chronosequence in northwestern Estonia. View in new window/tab. |
In each area three circular sample plots of 400 m2 (radius = 11.28 m) were established (all together 18 sample plots). Plots were randomly located and situated on average 375 m (min 70 m, max 1087 m) apart from each other. Plots belonged to Calluna, Cladina and Vaccinium uliginosum forest site types (Köster et al. 2016).
2.2 Data collection
In every circular sample plot a 10 × 10 m square (100 m2) vegetation plot was established. The sides of the vegetation plot were positioned parallel to cardinal directions. In every vegetation plot the lists of vascular plant and bryophyte species found were compiled following a similar methodology as described in Tullus et al. (2020). The cover of every species was evaluated visually following a 5-degree cover-abundance scale (1 = cover 1–5%, 2 = cover 6–20%, 3 = cover 21–50%, 4 = cover 51–75%, 5 = cover 76–100%; 0.5 was used for species covering less than 1%). The bryophyte survey included the examination of all possible growth substrates. Substrate types (soil, dead-wood, trunks and bases of pine and birch trees) was recorded and the lists of bryophyte species on different substrates were compiled. If the same species was found on multiple substrates (e.g., soil and dead-wood), the species was included in the lists for all substrates where it was present. Specimens that could not be identified in the field were collected for further investigation under a light microscope. The total cover of the vascular plant layer (including forbs, dwarf-shrubs and graminoids) and the total cover of the bryophyte layer (including ground-dwelling bryophytes) were also estimated visually, using a scale of 0–100%. Vegetation surveys were carried out in July 2020. The nomenclature follows Leht (2010) for vascular plants and Vellak et al. (2015) for bryophytes.
Hemispherical photos were taken at the height of the vascular plant layer during the vegetation surveys with Sigma’s 8mm F3.5 EX DG Circular Fisheye lens attached to a Canon EOS 6D Mark II digital camera. Photos were taken from five positions (centre, and four corners of each plot) and analysed using the software Gap Light Analyzer 2.0 (Frazer et al. 1999) to estimate the percentage of canopy openness and the amount of canopy-transmitted direct, diffuse, and total solar radiation. For further data analysis values of the five measurements were averaged.
2.3 Soil properties
Soils were sampled in the late spring of 2018. In each sample plot, 20 host trees were randomly selected. Below each tree, two soil samples were collected from opposite directions (1–1.5 m from the tree). A total of 40 soil cores (10 cm deep) were collected from each sample plot and composited into a single sample (all together 18 composited samples for analyses). Soil total nitrogen and carbon content were determined by Dumas’ method on a varioMAX CNS elemental analyzer (ELEMENTAR, Germany). Soil pH was measured in a suspension of soil with water and 1M KCl solution (1:2.5) using a pH meter, Mettler Toledo Seven Easy (Mettler Toledo. USA). Extractable Ca and Mg were determined by the Neutral Normal Ammonium Acetate (NNAA) method after extraction with 1M ammonium acetate solution at pH = 7.0. Plant available P and K in soils were determined using Egner-Riehm-Domingo method (Egner et al. 1960) with extraction by ammonium acetate (0.1 M) and acetic acid (0.4 M), pH = 3.7. Elemental contents in extracts were determined spectrometrically by microwave atomic emission using an MP-4200 (Agilent, USA).
2.4 Data analysis
Basal area for tree species in each sample plot was calculated based on data from Köster et al. (2016). Vascular plant species were grouped into shrubs and young trees (up to 4 m high), dwarf-shrubs (i.e., subshrubs with height < 1 m), graminoids, and forbs. The midpoint cover values of an abundance class were used for vascular plant and bryophyte species.
Shannon’s diversity index (H’) of vascular plants and bryophytes was estimated for each vegetation plot with PC-ORD Version 7 (McCune and Mefford 2016) as follows (Eq. 1):
![]()
where pi is the proportion of the sample belonging to the ith species.
Because of the intercorrelations among the seven soil variables (pH, N, P, K, C, Ca, Mg), Principal Component Analysis (PCA) was used to avoid multicollinearity. Two principal components (PC1, PC2) were used as explanatory variables in the models of vegetation characteristics; PC1 (explaining 74% of the variance, eigenvalue = 5.2) was significantly correlated with soil Ca (r = –0.83), Mg (r = –0.96), P (r = –0.94), K (r = –0.92), N (r = –0.93) and C (r = –0.99). PC2 (explaining 17% of the variance, eigenvalue = 1.2) was significantly correlated with soil pH (r = –0.97).
The effects on the cover of vascular plant and bryophyte layers of time since fire and environmental variables were analysed using linear mixed models (LMM), where stand was added as a random factor to account for possible spatial dependencies in data. Only total transmitted radiation was included into the models because light-related factors (canopy-transmitted direct, diffuse and total radiation, and canopy openness) were strongly intercorrelated (r > 0.9). Thus, the explanatory variables in the final models were time since fire, canopy transmitted total radiation, and the PC1 and PC2 scores. Time since fire and species richness estimates (1 was added, when the respective species data included zero values) were log-transformed before LMM analysis. The LMM analysis was carried out using function lmer from package lme4 in R Statistics software (R Core Team 2021). Backward variable selection was applied to obtain the final models, using function step from package lmerTest. The LMMs were run separately for the following dependent variables: the richness and diversity of vascular plants and bryophytes and the richness of the following groups: moss and liverwort species (including all recorded species), shrubs and young trees, dwarf-shrubs, graminoids, forbs, ground-dwelling bryophytes, epiphytic bryophytes on pine trees, epiphytic bryophytes on birch trees and bryophytes on dead-wood. Normality of the model residuals was checked visually from the Q-Q plots.
Two groups were distinguished, based on time since fire: 1) recently burned stands (ranging from 12 to 38 years since fire) and 2) stands with longer post-fire recovery periods (ranging from 69 to 183 years since fire). Compositional differences in vascular plant and bryophyte assemblages between these groups were tested with Multi-response Permutation Procedures (MRPP) using the Bray-Curtis distance measure and default options in PC-ORD 7. Indicator Species Analysis (ISA) was performed with PC-ORD 7 to find species characteristic of recently burned stands and stands with longer recovery period. Vascular plant and bryophyte assemblage data were ordinated with non-metric multidimensional scaling (NMDS), using the function “metaMDS” and Bray-Curtis dissimilarities in the community ecology package, Vegan in R (Oksanen et al. 2013). To interpret the ordination, environmental factors were fitted onto the ordination using the function “envfit”.
3 Results
3.1 Diversity of vascular plants and bryophytes
A total of 31 species of vascular plants and 39 species of bryophytes (9 liverwort and 30 moss species) were found in 18 vegetation plots (Supplementary file S1). In addition, two specimens of vascular plants and three specimens of bryophytes were identified only at the genus level due to the juvenility of specimens. Among vascular plants a dwarf-shrub Calluna vulgaris (L.) was the most frequent species, present in 94% of vegetation plots, followed by dwarf-shrubs Vaccinium vitis-idaea (L.) and V. myrtillus (L.) (present in 83% and 78% of plots respectively). Among bryophytes three moss species Dicranum scoparium Hedw., D. polysetum Sw. ex anon. and Pleurozium schreberi (Willd. ex Brid.) Mitt. were found in every vegetation plot. The majority of recorded species were common taxa, however, the protected vascular plant species Myrica gale (L.) and Goodyera repens (L.) R. Br. were found in one vegetation plot and two bryophyte species (Dicranum spurium Hedw. and Nowellia curvifolia (Dicks.) Mitt.) that are considered to be indicator species of woodland key habitats (Ministry of the Enivronment 2017) were recorded as well (N. curvifolia in two vegetation plots and D. spurium in one vegetation plot).
Altogether, 11 vascular plant and 19 bryophyte species were found in both stand groups (stands with 12–38 years since fire and stands with 69–183 years since fire). Recently burned stands contained 13 unique vascular plant and 10 bryophyte species (e.g., typical pioneer species such as Epilobium angustifolium (L.), Populus tremula (L.), Polytrichum juniperinum Hedw., P. piliferum Hedw. and Ceratodon purpureus (Hedw.) Brid.). Seven unique vascular plant and 10 bryophyte species were recorded in stands with a longer post-fire recovery period, including late-successional species such as G. repens and Calypogeia integristipula Steph.
According to the substrate type, 26% of bryophyte species inhabited only the soil surface, 10% grew on dead-wood, 8% on trunks and bases of birch trees, and 3% on trunks and bases of pine trees, whereas 54% of bryophyte species were found on multiple substrate types (Suppl. file S1).
3.2 The impact of environmental factors on floristic variables
3.2.1 The cover of vascular plant and bryophyte layers
The cover of the vascular plant layer showed high variation between vegetation plots, varying from 7% to 70%. The variation in the cover of the bryophyte layer was similarly high, ranging from 15% to 90%. The cover of both layers increased significantly with a longer time period since fire (Table 2). In addition, the cover of the vascular plant layer was positively correlated with canopy transmitted total radiation, being higher in more open stands.
| Table 2. Floristic variables in vegetation plots (n = 18) and the effects of environmental factors (standardized coefficients) on them, based on final linear mixed models after backward variable selection. PC1 and PC2 – principal components representing soil variables (detailed description of PCs is provided in the Data analysis section). | |||||||
| Response variable | Mean | Min | Max | Factor effect, standardized coefficient | |||
| Time since fire | Transmitted total radiation | PC1 | PC2 | ||||
| Cover of understorey vascular plant layer | 43.6 | 7 | 70 | 0.94 ± 0.24 | 0.75 ± 0.24 | - | - |
| Cover of bryophyte layer | 52.9 | 15 | 90 | 0.78 ± 0.16 | - | - | - |
| Richness of vascular plants | 7.8 | 5 | 13 | - | - | - | - |
| Shannon’s diversity index of vascular plants | 1.2 | 0.6 | 2.0 | - | - | - | - |
| Richness of shrubs and young trees | 1.8 | 0 | 4 | - | - | - | –0.61 ± 0.20 |
| Richness of dwarf-shrubs | 3.6 | 1 | 6 | 0.60 ± 0.20 | - | - | - |
| Richness of graminoids | 1.4 | 0 | 4 | - | –0.45 ± 0.17 | - | –0.65 ± 0.17 |
| Richness of forbs | 1.0 | 0 | 2 | - | - | - | - |
| Richness of bryophytes | 10.9 | 5 | 21 | - | 0.44 ± 0.16 | –0.61 ± 0.16 | –0.49 ± 0.15 |
| Shannon’s diversity index of bryophytes | 1.3 | 0.7 | 1.7 | - | - | - | –0.49 ± 0.22 |
| Richness of liverworts | 1.7 | 0 | 5 | –0.74 ± 0.16 | - | –0.61 ± 0.16 | - |
| Richness of mosses | 9.2 | 4 | 18 | - | - | –0.45 ± 0.18 | –0.57 ± 0.18 |
| Richness of ground-dwelling bryophytes | 8.3 | 3 | 18 | - | 0.42 ± 0.19 | - | –0.52 ± 0.19 |
| Richness of bryophytes on pine trees | 2.1 | 0 | 5 | - | –0.69 ± 0.18 | - | - |
| Richness of bryophytes on birch trees | 1.3 | 0 | 11 | - | - | - | - |
| Richness of bryophytes on dead-wood | 3.6 | 0 | 12 | - | - | –0.55 ± 0.15 | –0.59 ± 0.15 |
3.2.2 Species richness and diversity
Species richness and Shannon’s diversity index of vascular plants were unaffected by time since fire, according to the LMM results, however the richness of dwarf-shrubs was positively correlated with time since fire (Table 2). The richness of shrubs and young trees was negatively correlated with PC2, for which the main contributor was soil pH (r = –0.97), indicating that the number of woody species was higher on vegetation plots with higher soil pH. PC1, which correlated strongly with soil C, Mg, P, N, K and Ca, affected the richness of graminoids in the vegetation plots (i.e., more graminoids on more fertile plots).
The richness and diversity of bryophytes were impacted by soil conditions. Fertility (PC1) affected the richness of liverworts and mosses and the richness of bryophytes on dead-wood that were higher in more nutrient-rich sites, whereas PC2 (pH) had an effect on the diversity and richness of bryophytes, especially on mosses and on bryophytes on the ground and on dead-wood, indicating that higher soil pH was beneficial for the richness of these species groups. Ground-dwelling species and species growing on pine trees showed opposite responses towards light; higher amounts of light increased bryophyte species richness on the ground and decreased the number of species growing on pine trees. Liverworts was the only bryophyte group with richness negatively impacted by time since fire.
3.2.3 Species composition
Time since fire was significantly correlated with compositional differences between the assemblages of vascular plants and bryophytes, being also affected by light and soil conditions, according to the results of the NMDS ordination (Table 3, Fig. 2). Canopy openness and the amount of canopy transmitted light were high in the youngest stands where pioneer species (e.g., E. angustifolium, C. purpureus and P. piliferum) or species preferring open habitats (Carex arenaria (L.)) were recorded. At the same time, the compositional patterns for vascular plants were shaped by soil pH and the content of soil K and Ca, whereas bryophytes were affected by the content of soil N, P, K, C and Mg.
| Table 3. Relationships between vascular plant and bryophyte species composition (non-metric multidimensional scaling, Fig. 2) and environmental variables in a post-fire chronosequence in northwestern Estonia. R2 – correlation between environmental variable and ordination. P-values are based on random permutations of the data, significant relationships (p < 0.05) are shown in bold. | ||||
| Environmental variable | Vascular plants | Bryophytes | ||
| r2 | p-value | r2 | p-value | |
| Time since fire | 0.62 | 0.001 | 0.60 | 0.001 |
| Canopy transmitted direct radiance | 0.56 | 0.006 | 0.65 | 0.001 |
| Canopy transmitted diffuse radiance | 0.57 | 0.003 | 0.65 | 0.002 |
| Canopy transmitted total radiance | 0.57 | 0.004 | 0.65 | 0.001 |
| Canopy openness | 0.58 | 0.003 | 0.63 | 0.002 |
| soil pH | 0.36 | 0.033 | 0.30 | 0.064 |
| soil N | 0.13 | 0.383 | 0.38 | 0.023 |
| soil P | 0.25 | 0.121 | 0.47 | 0.007 |
| soil K | 0.33 | 0.045 | 0.53 | 0.004 |
| soil C | 0.25 | 0.112 | 0.49 | 0.006 |
| soil Ca | 0.34 | 0.046 | 0.19 | 0.219 |
| soil Mg | 0.28 | 0.081 | 0.36 | 0.033 |
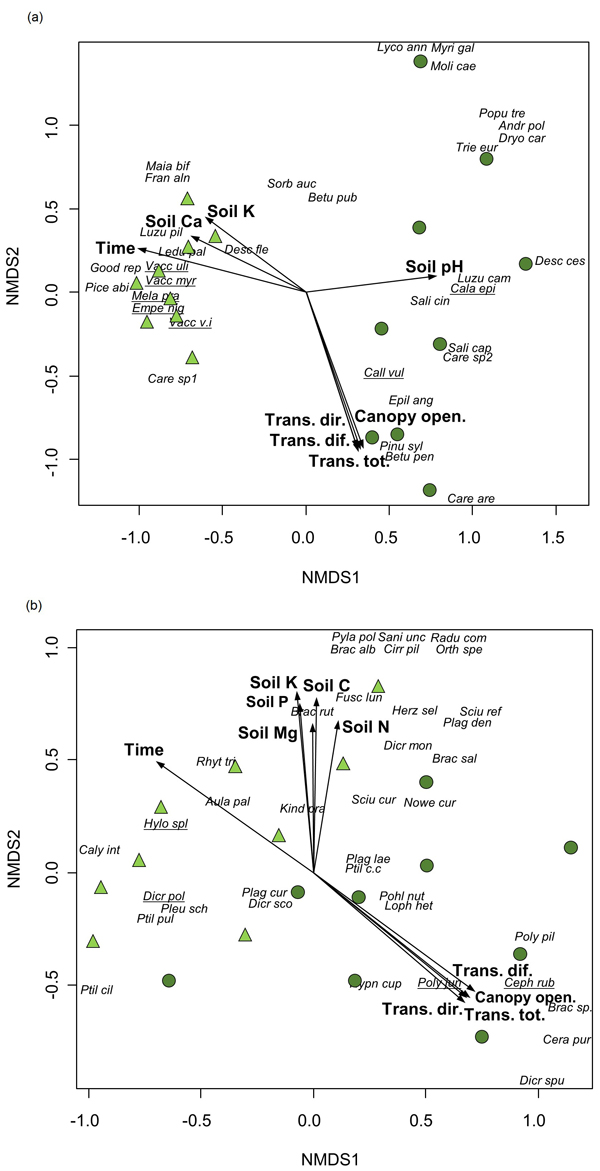
Fig. 2. Non-metric multidimensional scaling of a) vascular plants (2-dimensional solution, stress 0.12) and b) bryophytes (stress 0.14) from vegetation plots (n = 18) in a post-fire chronosequence in northwestern Estonia. Environmental factors that were significantly (p < 0.05) related to ordination axes (Table 3) are presented (Time – time since fire, Trans.dir. – canopy transmitted direct radiance, Trans.dif. – canopy transmitted diffuse radiance, Trans.tot. – canopy transmitted total radiance; Canopy open. – percentage of canopy openness, Soil pH – soil pH, Soil N – content of soil N, Soil P – content of soil P, Soil K – content of soil K, Soil C – content of soil C, Soil Ca – content of soil Ca, Soil Mg – content of soil Mg). Circles – vegetation plots in stands with 12 to 38 years since fire, triangles – vegetation plots in stands with 69 to 183 years since fire. Characteristic species of Indicator Species Analysis are underlined. Species abbreviations are provided in Supplementary file S1.
The significant effect of time since fire was also confirmed by MRPP in the composition of both vascular plants and bryophytes that differed considerably between groups; recently burned stands vs. stands with longer recovery period since fire (p < 0.001 for both taxonomic groups). In addition, ISA pointed out four species characteristic of recently burned stands: Calamagrostis epigeios (L.) Roth with indicator value 55.6, C. vulgaris (Indicator species value = 77.8), Cephaloziella rubella (Nees) Warnst. (55.6) and P. juniperinum (100.0), and seven species in stands with longer recovery period: Empetrum nigrum (L.) (84.4), Melampyrum pratense (L.) (86.8), V. myrtillus (90.6), V. uliginosum (55.6), V. vitis-idaea (81.5), D. polysetum (67.8) and Hylocomium splendens (Hedw.) Schimp. (87.7).
4 Discussion
The aim of our study was to analyse the effects of fire disturbance on vascular plants and bryophytes in a chronosequence of Scots pine stands, and to identify the underlying environmental factors affecting the post-fire dynamics of species diversity, cover, and composition. Our study revealed that time since the last forest fire affected the compositional patterns of vascular plants and bryophytes, whereas the impacts of fire disturbance on species richness and diversity estimates were less evident. The cover of vascular plant and bryophyte layers increased over time, similar to the positive relationship between stand age and understorey cover observed by Kumar et al. (2018b).
Other studies have shown that fires may have favourable effects on the richness of understorey vegetation in hemiboreal and boreal forests (Wang and Kemball 2005; Marozas et al. 2007; Ruokolainen and Salo 2009). The increased understorey species richness shortly after fire may be explained by the influx of pioneer species and quick recovery of species dominant pre-fire. For example Marozas et al. (2007) demonstrated that early successional species invaded burned areas 1–3 years after fire, whereas the recovery of dwarf-shrubs and herbs that dominated the pre-fire understorey vegetation was observed already 5–6 years after surface fire in Scots pine forests of hemiboreal Lithuania. However, in our chronosequence the shortest time since last forest fire was already 12 years and therefore the study skips the early period when species diversity and biomass may fluctuate strongly after stand-replacing fires (Skre et al. 1998; Parro et al. 2009; Marozas et al. 2013; Fornwalt and Kaufmann 2014) and some stabilization of plant communities had already occurred, although some pioneer species were still present. Therefore, it was not surprising that time since fire in our study did not influence the richness and diversity of vascular plants and bryophytes or the richness of most species groups.
Nevertheless, the negligible effect of time since fire on the richness of these species groups could be related to shifts in composition during post-fire vegetation succession. For example Jean et al. (2017) pointed out that around 20 to 40 years post-fire, mid-to late-successional species replaced pioneer species. In our study, both stand types (post-fire intervals) contained unique vascular plant and bryophyte species and compositional differences between them were also confirmed by ISA and MRPP. C. epigeios, C. vulgaris, C. rubella and P. juniperinum were characteristic of recently burned stands and E. nigrum, M. pratense, V. myrtillus, V. ulignosum, V. vitis-idaea, D. polysetum and H. spendens were indicator species to stands with a longer recovery period. This is accordance with results by Gloaguen (1990) and Adámek et al. (2016), who also found that C. vulgaris and Polytrichum spp. dominated stands in the first 5–13 years after fire. Similarly, C. vulgaris and P. juniperum were the most abundant species in Latvia in the first 6–7 years after fire in P. sylvestris stands (Purina et al. 2016). In addition, Parro et al. (2009) found that shortly after forest-fire C. epigeios, C. vulgaris and P. juniperinum were favored by forest fire.
In other boreal forests, C. rubella is of recently burned stands, for example in Canada (Paquette et al. 2016) and in Finland where the species often has been found to grow on charred organic material as well (Ryömä and Oldén 2013). The dominance of the dwarf-shrub V. myrtillus and a pleurocarpous moss H. splendens is expected later in post-fire succession (Skre et al. 1998; Jean et al. 2019). Grandpré et al. (1993) showed that H. splendens appeared 46 years after fire on burned sites in Canadian boreal forests. Although H. splendens was present in recently burned stands in our study, it had very low cover. Another characteristic species to stands with longer recovery period – D. polysetum – was present in every sample plot of our study but its cover was remarkably higher in stands with a longer recovery period (Suppl. file S1), in accordance with Parro et al. (2009) who showed the destructive effect of fire on the cover of D. polysetum referring to the longer recovery period necessary for this species.
Soil variables were important drivers for the richness of understorey vegetation groups. PC2, indicative of soil pH, was positively correlated with the richness of shrubs and young trees and graminoids as well as with the richness of bryophytes, mosses, epigeic and epixylic bryophytes. In general the soil pH of the studied sites was very low, ranging from 2.1 to 3.3, therefore the higher richness of several plant groups on sites with higher pH can be expected and it is in accordance with several former studies (Pausas 1994; Olden et al. 2016). The richness of bryophytes (both mosses and liverworts as well as epixylic bryophytes) was higher in sites with higher soil nutrient content (PC2) that is also in accordance with expectations for plant communities on nutrient poor soils, similar to outcomes from a study of vegetation patterns in nutrient poor pine forests managed with low-intensity harvesting methods in south-western Estonia (Tullus et al. 2020).
The only species groups for which richness was significantly affected by time since fire were the dwarf-shrubs and liverworts that showed opposite responses to a longer period since fire. The higher number of liverworts in recently burned stands may seem suprising, as the richness of liverworts is known to benefit from a moist microclimate (Fenton and Frego 2005) that is uncharacteristic of recently burned sites. However, in the current study, the higher number of liverworts in recently burned stands was due to the presence of a species of genus Chephaloziella belonging to the life strategy group of colonists that are typical to disturbed habitats (Glime 2017).
The total richness of bryophytes and specifically the richness of epigeic bryophytes increased in more open stands. As bryophytes are usually regarded as shade tolerant plants (Humphrey et al. 2002; Ódor et al. 2014), this was somewhat unexpected, however it may be explained by the presence of a pioneer bryophyte species in more open stands that increased the bryophyte diversity. The richness of bryophytes on pine trees was higher in more shaded stands, although we did not detect differences in the response of bryophyte communities on different substrate types with respect to the time since fire.
Historically fire has been one of the main disturbances in boreal and hemiboreal forest ecosystems (Stocks et al. 1998; Bond-Lamberty et al. 2007; Donis et al. 2017; Drobyshev et al. 2021), however nowadays logging is also an important disturbance in these forest ecosystems (Kuuluvainen et al. 2021). Although the long-term effects of forest fires and logging on understorey vegetation are often believed to be similar, it has not been wisely tested especially in the forests of Northern Europe (but see Paquette et al. 2016; Rowe et al. 2017; Jean et al. 2019 for comparisons to forests of North America). When results of our fire chronosequence were compared with the results of a study in dry boreal Scots pine forests managed with low-intensity methods (Tullus et al. 2020), the species richness of bryophytes was similar in both fire-origin and logged stands (average richness of bryophytes 10.9 and 10.8, respectively). At the same time the richness of vascular plants was slightly higher in logged stands, being on average 10.6 for logged stands and 7.8 for fire-origin stands. In addition, species characteristic of recently burned stands (C. vulgaris, C. epigeios, C. rubella and P. juniperinum) were present also in logged stands, however with considerably lower cover. While this comparison indicates that the long-term effects of fire on vascular plants and bryophytes may be quite similar with logging-induced effects, it comes with a caveat. The stands harvested with low-intensity logging methods that were found to be beneficial for the overall richness of vascular plants and bryophytes were in nutrient poor pine forests (Tullus et al. 2018, 2019), thus these results cannot be extrapolated to high-intensity harvesting methods, such as clear-cutting. Hence, the long-term effects of clear-cutting in comparison with fire to the forests of Northern Europe remain to be studied more thoroughly. Another aspect that should be assessed is the impact of salvage logging on the biodiversity in post-fire stands.
5 Conclusions
As expected, we observed considerable compositional differences in vascular plant and bryophyte assemblages between stands recently burned (12–38 years) and stands burned a long-time ago (69–183 years). The trends for species richness along the post-fire chornosequence were less clear. Liverworts were the only species group with higher species richness in recently burned stands, due to the presence of Cepahloziella spp. in these stands. Although the long-term impacts of forest-fires and clear-cutting on forest biodiversity are often claimed to have similar effects, these disturbances have not been thoroughly studied in the region and future studies should compare their long-term as well as short-term effects.
Authors’ contributions
Conceptualization – T.T., M.M., A.O. and K.K; methodology – T.T., M.M. and A.O.; data collection and analysis – T.T., A.O. and A.T.; writing – original draft preparation – A.O., T.T., A.T. and M.M.; writing – review and editing – A.O., T.T., A.T., M.M. and K.K. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
We would like to thank John A. Stanturf for language editing. This work was supported by the Estonian Research Council grant (PRG1586), projects of the Estonian University of Life Sciences (P180024MIME, P200029MIME), Academy of Finland (294600) and the European Union, European Regional Development Fund (Estonian University of Life Sciences ASTRA project “Value-chain based bioeconomy”).
Declaration of openness of research materials, data, and code
All materials and data are available upon request from corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
References
Adámek M, Hadincová V, Wild J (2016) Long-term effect of wildfires on temperate Pinus sylvestris forests: Vegetation dynamics and ecosystem resilience. Forest Ecol Manag 380: 285–295. https://doi.org/10.1016/j.foreco.2016.08.051.
Bartels SF, Chen HYH (2015) Dynamics of epiphytic macrolichen abundance, diversity and composition in boreal forest. J Appl Ecol 52: 181–189. https://doi.org/10.1111/1365-2664.12360.
Bond-Lamberty B, Peckham SD, Ahl DE, Gower ST (2007) Fire as the dominant driver of central Canadian boreal forest carbon balance. Nature 450: 89–92. https://doi.org/10.1038/nature06272.
Brais S, Camiré C, Bergeron Y, Paré D (1995) Changes in nutrient availability and forest floor characteristics in relation to stand age and forest composition in the southern part of the boreal forest of northwestern Quebec. Forest Ecol Manag 76: 181–189. https://doi.org/10.1016/0378-1127(95)03541-H.
Certini G (2005) Effects of fire on properties of forest soils: a review. Oecologia 143: 1–10. https://doi.org/10.1007/s00442-004-1788-8.
Connell J, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111: 1119–1144. https://doi.org/10.1086/283241.
Donis J, Kitenberga M, Snepsts G, Matisons R, Zarins J, Jansons A (2017) The forest fire regime in Latvia during 1922–2014. Silva Fenn 51, article id 7746. https://doi.org/10.14214/sf.7746.
Drobyshev I, Ryzhkova N, Eden J, Kitenberga M, Pinto G, Lindberg H, Krikken F, Yermokhin M, Bergeron Y, Kryshen A (2021) Trends and patterns in annually burned forest areas and fire weather across the European boreal zone in the 20th and early 21st centuries. Agr Forest Meteorol 306, article id 108467. https://doi.org/10.1016/j.agrformet.2021.108467.
Egner H, Riehm H, Domingi WR (1960) Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nahrstoffzustandes der Boden, II : Chemische Extractionsmetoden zu Phosphor‐ und Kaliumbestimmung. [Investigations on soilchemical analysis as a basis of the evaluation of plant nutrient status of soils II. Chemical extraction methods for phosphorus and potassium determination]. Kungliga Lantbrukshügskolans Annaler 26: 199–215.
Estonian Weather Service (2020) https://www.ilmateenistus.ee/kliima/kliimanormid/ohutemperatuur/. Accessed 20 April 2021.
Fenton NJ, Frego KA (2005) Bryophyte (moss and liverwort) conservation under remnant canopy in managed forests. Biol Conserv 122: 417–430. https://doi.org/10.1016/j.biocon.2004.09.003.
Flannigan MD, Krawchuk MA, de Groot WJ, Wotton BM, Gowman LM (2009) Implications of changing climate for global wildland fire. Int J Wildland Fire 18: 483–507. https://doi.org/10.1071/WF08187.
Fornwalt PJ, Kaufmann MR (2014) Understorey plant community dynamics following a large, mixed severity wildfire in a Pinus ponderosa–Pseudotsuga menziesii forest, Colorado, USA. J Veg Sci 25: 805–818. https://doi.org/10.1111/jvs.12128.
Franklin JF, Spies TA, Pelt RV, Carey AB, Thornburgh DA, Berg DR, Lindenmayer DB, Harmon ME, Keeton WS, Shaw DC, Bible K, Chen J (2002) Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. Forest Ecol Manag 155: 399–423. https://doi.org/10.1016/S0378-1127(01)00575-8.
Frazer GW, Canham CD, Lertzman KP (1999) Gap Light Analyzer (GLA), version 2.0: imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies, Millbrook, New York. https://www.caryinstitute.org/science/our-scientists/dr-charles-d-canham/gap-light-analyzer-gla.
Glime M (2017) Chapter 4 – Adaptive strategies. Bryophyte Ecology Volume 1: Physiological Ecology 3. https://digitalcommons.mtu.edu/bryophyte-ecology1/3.
Gloaguen JC (1990) Post-burn succession on Brittany heathlands. J Veg Sci 1: 147–152. https://doi.org/10.2307/3235653.
Gorshkov VV, Bakkal IJ (1996) Species richness and structure variations of Scots pine forest communities during the period from 5 to 210 years after fire. Silva Fenn 30: 329–340. https://doi.org/10.14214/sf.a9233.
Grandpré L, Gagnon D, Bergeron Y (1993) Changes in the understory of Canadian southern boreal forest after fire. J Veg Sci 4: 803–810. https://doi.org/10.2307/3235618.
Halpern CB, Lutz JA (2013) Canopy closure exerts weak controls on understory dynamics: a 30-year study of overstory–understory interactions. Ecol Monogr 83: 221–237. https://doi.org/10.1890/12-1696.1.
Hart SA, Chen HYH (2006) Understory vegetation dynamics of North American boreal forests. Crit Rev Plant Sci 25: 381–397. https://doi.org/10.1080/07352680600819286.
Hart SA, Chen HYH (2008) Fire, logging, and overstory affect understory abundance, diversity, and composition in boreal forest. Ecol Monogr 78: 123–140. https://doi.org/10.1890/06-2140.1.
Humphrey JW, Davey S, Peace AJ, Ferris R, Harding K (2002) Lichens and bryophyte communities of planted and semi-natural forests in Britain: the influence of site type, stand structure and deadwood. Biol Conserv 107: 165–180. https://doi.org/10.1016/S0006-3207(02)00057-5.
Jean M, Alexander HD, Mack MC, Johnstone JF (2017) Patterns of bryophyte succession in a 160-year chronosequence in deciduous and coniferous forests of boreal Alaska. Can J Forest Res 47: 1021–1032. https://doi.org/10.1139/cjfr-2017-0013.
Jean M, Lafleur B, Fenton NJ, Paré D, Bergeron Y (2019) Influence of fire and harvest severity on understory plant communities. Forest Ecol Manag 436: 88–104. https://doi.org/10.1016/j.foreco.2019.01.004.
Jõgiste K, Korjus H, Stanturf JA, Frelich LE, Baders E, Donis J, Jansons A, Kangur A, Köster K, Laarmann D, Maaten T, Marozas V, Metslaid M, Nigul K, Polyachenko O, Randveer T, Vodde F (2017) Hemiboreal forest: natural disturbances and the importance of ecosystem legacies to management. Ecosphere 8, article id e01706. https://doi.org/10.1002/ecs2.1706.
Johnson EA, Miyanishi K (2008) Testing the assumptions of chronosequences in succession. Ecol Lett 11: 419–431. https://doi.org/10.1111/j.1461-0248.2008.01173.x.
Johnstone JF, Kasischke ES (2005) Stand-level effects of soil burn severity on postfire regeneration in a recently burned black spruce forest. Can J Forest Res 35: 2151–2163. https://doi.org/10.1139/x05-087.
Köster K, Köster E, Orumaa A, Parro K, Jõgiste K, Berninger F, Pumpanen J, Metslaid M (2016) How time since forest fire affects stand structure, soil physical-chemical properties and soil CO2 efflux in hemiboreal Scots pine forest fire chronosequence? Forests 7, article id 201. https://doi.org/10.3390/f7090201.
Kumar P, Chen HYH, Thomas SC, Shahi C (2017) Effects of coarse woody debris on plant and lichen species composition in boreal forests. J Veg Sci 28: 389–400. https://doi.org/10.1111/jvs.12485.
Kumar P, Chen HYH, Thomas SC, Shahi C (2018a) Epixylic vegetation abundance, diversity, and composition vary with coarse woody debris decay class and substrate species in boreal forest. Can J Forest Res 48: 399–411. https://doi.org/10.1139/cjfr-2017-0283.
Kumar P, Chen HYH, Thomas SC, Shahi C (2018b) Linking resource availability and heterogeneity to understorey species diversity through succession in boreal forest of Canada. J Ecol 106: 1266–1276. https://doi.org/10.1111/1365-2745.12861.
Kuuluvainen T (2002) Introduction. Disturbance dynamics in boreal forests: defining the ecological basis of restoration and management of biodiversity. Silva Fenn 36: 5–11. https://doi.org/10.14214/sf.547.
Kuuluvainen T, Mäki J, Karjalainen L, Lehtonen H (2002) Tree age distributions in old-growth forest sites in Vienansalo wilderness, eastern Fennoscandia. Silva Fenn 36: 169–184. https://doi.org/10.14214/sf.556.
Kuuluvainen T, Angelstam P, Frelich L, Jõgiste K, Koivula M, Kubota Y, Lafleur B, Macdonald E (2021) Natural disturbance-based forest management: moving beyond retention and continuous-cover forestry. Front For Glob Change 4, article id 629020. https://doi.org/10.3389/ffgc.2021.629020.
Leht M (ed) (2010) Eesti taimede määraja. [The key book of Estonian vascular plants]. EMÜ, Eesti Loodusfoto, Tartu.
Liu B, Yang J, Johnstone JF (2017) Understory vascular plant community assembly in relation to time-since-fire and environmental variables in a Chinese boreal forest. J Mt Sci 14: 1317–1328. https://doi.org/10.1007/s11629-016-4158-1.
Liu B, Biswas SR, Yang J, Liu Z, He HS, Liang Y, Lau MK, Fang Y, Han S (2020) Strong influences of stand age and topography on post-fire understory recovery in a Chinese boreal forest. For Ecol Manag 473, article id 118307. https://doi.org/10.1016/j.foreco.2020.118307.
Marozas V, Racinskas J, Bartkevicius E (2007) Dynamics of ground vegetation after surface fires in hemiboreal Pinus sylvestris forests. For Ecol Manag 250: 47–55. https://doi.org/10.1016/j.foreco.2007.03.008.
Marozas V, Armolaitis K, Aleinikovienė J (2013) Changes of ground vegetation, soil chemical properties and microbiota following the surface fires in Scots pine forests. J Environ Eng Landsc 21: 67–75. https://doi.org/10.3846/16486897.2012.663087.
McCune B, Mefford MJ (2016) PC-ORD. Multivariate analysis of ecological data. Version 7. MjM Software Design, Gleneden Beach, Oregon, USA.
Mills SE, Macdonald SE (2005) Factors influencing bryophyte assemblage at different scales in the Western Canadian boreal forest. Bryologist 108: 86–100. https://doi.org/10.1639/0007-2745(2005)108[86:FIBAAD]2.0.CO;2.
Ministry of the Enivronment (2017) Vääriselupaiga klassifikaator, valiku juhend, kaitse korraldamine ning vääriselupaiga kaitseks lepingu sõlmimine ja kasutusõiguse tasu arvutamise täpsustatud alused. [The classification of key habitats, guidelines for the selection and protection of key habitats]. Riigi Teataja. RT I, 15.09.2017, 10. https://www.riigiteataja.ee/akt/115092017010. Accessed 25 November 2020.
Ódor P, Király I, Tinya F, Bortignon F, Nascimbene J (2014) Patterns and drivers of species composition of epiphytic bryophytes and lichens in managed temperate forests. For Ecol Manag 321: 42–51. https://doi.org/10.1016/j.foreco.2014.01.035.
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Vegan: community ecology package.Rpackageversion 2.0-10. http://CRAN.R-project.org/package=vegan.
Oldén A, Raatikainen KJ, Tervonen K, Halme P (2016) Grazing and soil pH are biodiversity drivers of vascular plants and bryophytes in boreal wood-pastures. Agric Ecosyst Environ 222: 171−184. https://doi.org/10.1016/j.agee.2016.02.018.
Paquette M, Boudreault C, Fenton N, Pothier D, Bergeron Y (2016) Bryophyte species assemblages in fire and clear-cut origin boreal forests. For Ecol Manag 359: 99–108. https://doi.org/10.1016/j.foreco.2015.09.031.
Pare D, Bergeron Y (1996) Effect of colonizing tree species on soil nutrient availability in a clay soil of the boreal mixedwood. Can J Forest Res 26: 1022–1031. https://doi.org/10.1139/x26-113.
Parro K, Köster K, Jõgiste K, Vodde F (2009) Vegetation dynamics in a fire damaged forest area: the response of major ground vegetation species. Balt For 15: 206–215.
Pausas JG (1994) Species richness patterns in the understorey of Pyrenean Pinus sylvestris forest. J Veg Sci 5: 517–524. https://doi.org/10.2307/3235978.
Purina L, Straupe I, Liepa L, Libiete Z, Zadina M, Jansons A (2016) Long-term influence of large forest fire on ground vegetation. In: ‘Research for Rural Development’ 18–20 May 2016, Latvia University of Agriculture, Jelgava, pp 28–33.
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Ribeiro-Kumara C, Pumpanen J, Heinonsalo J, Metslaid M, Orumaa A, Jõgiste K, Berninger F, Köster K (2019) Long-term effects of forest fires on soil greenhouse gas emissions and extracellular enzyme activities in a hemiboreal forest. Sci Total Environ 718, article id 135291. https://doi.org/10.1016/j.scitotenv.2019.135291.
Rowe ER, D’Amato AW, Palik BJ, Almendinger JC (2017) Early response of ground layer plant communities to wildfire and harvesting disturbance in forested peatland ecosystems in northern Minnesota, USA. For Ecol Manag 398: 140–152. https://doi.org/10.1016/j.foreco.2017.05.012.
Ruokolainen L, Salo K (2009) The effect of fire intensity on vegetation succession on a sub-xeric heath during ten years after wildfire. Ann Bot Fenn 46: 30–42. https://doi.org/10.5735/085.046.0103.
Ryömä R, Oldén A (2013) Suomen puutteellisesti tunnetut maksasammalsuvut: siiransammalet Nardia, rahtusammalet Cephaloziella ja pihtisammalet Cephalozia. [The poorly known liverwort genera Nardia, Cephaloziella and Cephalozia in Finland]. http://hdl.handle.net/10138/39579.
Schimmel J, Granstrom A (1996) Fire severity and vegetation response in the Boreal Swedish Ecology 77: 1436–1450. https://doi.org/10.2307/2265541.
Seidl R, Fernandes PM, Fonseca TF, Gillet F, Jönsson AM, Merganičová K, Netherer S, Arpaci A, Bontemps J-D, Bugmann H, González-Olabarria JR, Lasch P, Meredieu C, Moreira F, Schelhaas M-J, Mohren F (2011) Modelling natural disturbances in forest ecosystems: a review. Ecol Model 222: 903–924. https://doi.org/10.1016/j.ecolmodel.2010.09.040.
Simard DG, Fyles JW, Paré D, Nguyen T (2001) Impacts of clearcut harvesting and wildfire on soil nutrient status in the Quebec boreal forest. Can J Soil Sci 81: 229–237. https://doi.org/10.4141/S00-028.
Skre O, Wielgolaski FE, Moe B (1998) Biomass and chemical composition of common forest plants in response to fire in western Norway. J Veg Sci 9: 501–510. https://doi.org/10.2307/3237265.
Stocks BJ, Fosberg MA, Lynham TJ, Mearns L, Wotton BM, Yang Q, Jin J-Z, Lawrence K, Hartley GR, Mason JA, McKenney DW (1998) Climate change and forest fire potential in Russian and Canadian boreal forests. Climatic Change 38: 1–13. https://doi.org/10.1023/A:1005306001055.
Tullus T, Rosenvald R, Leis M, Lõhmus P (2018) Impacts of shelterwood logging on forest bryoflora: distinct assemblages with richness comparable to mature forests. For Ecol Manag 411: 67–74. https://doi.org/10.1016/j.foreco.2018.01.008.
Tullus T, Tishler M, Rosenvald R, Tullus A, Lutter R, Tullus, H (2019) Early responses of vascular plant and bryophyte communities to uniform shelterwood cutting in hemiboreal Scots pine forests. For Ecol Manag 440: 70–78. https://doi.org/10.1016/j.foreco.2019.03.009.
Tullus T, Lutter R, Randlane T, Saag A, Tullus A, Roosaluste E, Kõresaar P, Pärtel M, Tullus H (2020) Seventy-year history of management using low-intensity harvesting methods: weak impact on biodiversity of hemiboreal Scots pine forests. Can J Forest Res 50: 1268–1280. https://doi.org/10.1139/cjfr-2020-0102.
Úbeda X, Sarricolea P (2016) Wildfires in Chile: a review. Global Planet Change 146: 152–161. https://doi.org/10.1016/j.gloplacha.2016.10.004.
Vellak K, Ingerpuu N, Leis M, Ehrlich L (2015) Annotated checklist of Estonian bryophytes. Folia Cryptogam Est 52: 109–127. https://doi.org/10.12697/fce.2015.52.14.
Walker LR, Wardle DA, Bardgett RD, Clarkson BD (2010) The use of chronosequences in studies of ecological succession and soil development: chronosequences, succession and soil development. J Ecol 98: 725–736. https://doi.org/10.1111/j.1365-2745.2010.01664.x.
Walker XJ, Baltzer JL, Cumming SG, Day NJ, Ebert C, Goetz S, Johnstone JF, Potter S, Rogers BM, Schuur EAG, Turetsky MR, Mack MC (2019) Increasing wildfires threaten historic carbon sink of boreal forest soils. Nature 572: 520–523. https://doi.org/10.1038/s41586-019-1474-y.
Wang GG, Kemball KJ (2005) Effects of fire severity on early development of understory vegetation. Can J Forest Res 35: 254–262. https://doi.org/10.1139/x04-177.
Total of 69 references.

