Effect of climatic factors on tree-ring width of Populus hybrids in Latvia
Šēnhofa S., Zeps M., Matisons R., Smilga J., Lazdiņa D., Jansons Ā. (2015). Effect of climatic factors on tree-ring width of Populus hybrids in Latvia. Silva Fennica vol. 50 no. 1 article id 1442. https://doi.org/10.14214/sf.1442
Highlights
- Hybrid poplar and hybrid aspen were sensitive to temperature in summer and dormant periods, but none of the tested factors were strictly limiting
- Hybrid poplar was sensitive to a higher number of climatic factors than hybrid aspen
- Temperature showed a negative correlation with tree-ring width.
Abstract
Fast-growing hybrids of Populus L. have an increasing importance as a source of renewable energy and as industrial wood. Nevertheless, the long-term sensitivity of Populus hybrids to weather conditions and hence to possible climatic hazards in Northern Europe have been insufficiently studied, likely due to the limited age of the trees (short rotation). In this study, the climatic sensitivity of ca. 65-year-old hybrid poplars (Populus balsamifera L. × P. laurifolia Ledeb.), growing at two sites in the western part of Latvia, and ca. 55-year-old hybrid aspens (Populus tremuloides Michx. × P. tremula L.), growing in the eastern part of Latvia, have been studied using classical dendrochronological techniques. The high-frequency variation of tree-ring width (TRW) of hybrid poplar from both sites was similar, but it differed from hybrid aspen due to the diverse parental species and geographic location of the stands. Nevertheless, some common tendencies in TRW were observed for both hybrids. Climatic factors influencing TRW were generally similar for both hybrids, but their composition differed. The strength of climate-TRW relationships was similar, but the hybrid poplar was affected by a higher number of climatic factors. Hybrid poplar was sensitive to factors related to water deficit in late summer in the previous and current years. Hybrid aspen was sensitive to conditions in the year of formation of tree-ring. Both hybrids also displayed a reaction to temperature during the dormant period. The observed climate-growth relationships suggest that increasing temperatures might burden the radial growth of the studied hybrids of Populus.
Keywords
dendroclimatology;
hybrid aspen;
hybrid poplar;
fast-growing hybrids;
weather conditions
- Šēnhofa, LSFRI “Silava”, Rigas str. 111, Salaspils, Latvia, LV2169 E-mail silva.senhofa@gmail.com
- Zeps, LSFRI “Silava”, Rigas str. 111, Salaspils, Latvia, LV2169 E-mail martins.zeps@silava.lv
-
Matisons,
LSFRI “Silava”, Rigas str. 111, Salaspils, Latvia, LV2169
E-mail
robism@inbox.lv

- Smilga, LSFRI “Silava”, Rigas str. 111, Salaspils, Latvia, LV2169 E-mail janis.smilga@silava.lv
- Lazdiņa, LSFRI “Silava”, Rigas str. 111, Salaspils, Latvia, LV2169 E-mail dagnija.lazdina@silava.lv
- Jansons, LSFRI “Silava”, Rigas str. 111, Salaspils, Latvia, LV2169 E-mail aris.jansons@silava.lv
Received 21 August 2015 Accepted 29 October 2015 Published 11 November 2015
Views 148748
Available at https://doi.org/10.14214/sf.1442 | Download PDF

1 Introduction
Growing demand for timber and renewable energy from biomass (Schueler et al. 2013) is increasing the importance of Populus L. hybrids, which are known for high productivity (Yu et al. 2001; Tullus et al. 2013). In the Baltic Sea region, Populus hybrids appear especially promising for use on abandoned agricultural lands (Christersson 2007; Tullus et al. 2011, 2013). Still, before wider application of any planting materials, possible interactions with the environment should be comprehensively evaluated. Considering that climate is one of the main factors affecting tree growth and productivity of forest ecosystems (Kirschbaum 2000; Lindner et al. 2010), increments of Populus hybrids might be altered due to changes of climate in the future. This has been suggested by the significant short-term (intra-annual) relationships observed between meteorological factors and increment of young Populus hybrids (Yu et al. 2001; Tullus et al. 2011; Jansons et al. 2014). Nevertheless, the long-term (inter-annually) variation of increment might be affected by distinct climatic factors compared to that observed for intra-annual variation (Hughes and Funkhouser 2003; Čufar et al. 2008; Seo et al. 2011), and such knowledge might be crucial for sufficient and sustainable management (Burton 2012).
Detailed information about the effects of climatic factors on tree growth in a long-term can be obtained via retrospective analysis of tree-ring parameters by applying dendrochronological techniques (Fritts 2001). The most common parameter for such studies is tree-ring width (TRW) (Speer 2010), although radial increment is formed during a certain part of the growing period (Yu et al. 2001; Deslauriers et al. 2009) and it might be affected by several factors (Cook 1992). Still, there is poor knowledge regarding long-term climate-growth relationships for Populus hybrids, likely due to the short rotation (20–30 years) period (Tullus et al. 2013) and hence the young age of trees, which is not sufficient for a dendrochronological analysis (Cook 1992; Fritts 2001). In Latvia, many experimental plantations of hybrid poplar and aspen were established around the 1960s (Mangalis 2004), and some of them have remained as long-term experiments until today. These plantations have provided a unique opportunity to study long-term variation in the radial growth of Populus hybrids. Therefore, the aim of this study was to assess the relationships between climatic factors and TRW of hybrid poplar (Populus balsamifera L. × P. laurifolia Ledeb.) and hybrid aspen (Populus tremuloides Michx. × P. tremula L.), which were older than 50 and 60 years, respectively. Considering the rapid growth and high transpiration rates of these hybrids, we assumed that factors related to the availability of water during the growing period have mainly affected their growth.
2 Materials and methods
2.1 Study areas, sampling, and measurements
Hybrid poplars (no additional information about the origin was available) were sampled in two experimental plantations in the western part of Latvia near Auce (AUC) (56°31´N, 22°56´E) and Šķēde (SKD) (57°14´N, 22°37´E). Hybrid aspens, the progenies of P. tremuloides growing in a botanical garden in the central part of Latvia (no information on the origin was available) and 10 local Populus tremula plus trees from the eastern part of Latvia, were sampled in one plantation in the eastern part of Latvia near Jaunkalsnava (KLN) (56°41´N, 25°54´E). The ages of the AUC, SKD and KLN plantations were 63, 68 and 56, respectively. The initial spacing of the trees at all sites was 3 × 3 m. The elevations of the studied stands were low, about 70, 110, and 100 m a.s.l. at the AUC, SKD, and KLN sites, respectively. The topography of all stands was flat. The studied stands were growing on fertile loamy soil under normal moisture conditions (corresponding to the Oxalidosa forest type). The climate in the studied sites was mild due to dominant winds, which bring cool and moist air masses from the Baltic Sea and the Atlantic; hence, the continentality of the climate was stronger in the eastern site. The mean monthly temperature ranged from ca. –3.5 and –6.0 to ca. +15.4 and +16.8 °C in January and July (the mean annual temperature was ca. +6 and +5 °C) in the sites in the western and eastern part of Latvia, respectively. The growing period usually extends from mid-April to mid-October. The mean annual precipitation in all sites was ca. 620 mm. The highest monthly precipitation occurs in the summer months (May–September), which usually results in a positive water balance throughout the year (Klavins and Rodinov 2010). A stable snow cover usually forms beginning with the last week of December, and it lasts until the first week of April. Climatic changes are mainly expressed as an increase of temperature in the autumn to spring period and as an extension of the growing period (Lizuma et al. 2007), while the precipitation regime in the summer is becoming more variable (Avotniece et al. 2010).
Visually healthy trees representing the diameter distribution of the plantations were selected according to trial inventories and felled in November and December of 2013. In total, 26 hybrid poplars (13 trees from each site) and 22 hybrid aspens were sampled. From each log, a stem disk at 1.3 m above the root collar was taken. In the laboratory, the surface of air-dried stem disks was gradually grinded with sandpaper (80, 120, 240 and 400 grains per inch). Tree-ring width was measured using the Lintab 5 (RinnTECH, Germany, Heidelberg) measurement system with the precision of 0.01 mm along two opposite radii of the stem, avoiding the reaction wood.
2.2 Data analysis
The time series of TRW were crossdated, and the quality was checked by a graphical inspection and statistically using the program COFECHA (Grissino-Mayer 2001). The time series, which showed low agreement with the rest of the dataset (r < 0.40), were rejected from further analysis rather than corrected. The statistics of the crossdated datasets, expressed population signal (EPS) (Wigley et al. 1984), Gleichläufigkeit (GLK), mean interseries correlation, signal to noise ratio (SNR) and first order autocorrelation, for the detrended time series were calculated in program R using the library “dplR” (Bunn 2008). Residual chronologies of TRW were established using the program ARSTAN (Cook and Holmes 1986). Double detrending by a negative exponential curve and cubic spline with rigidity of 40 years was applied and autocorrelation was removed. The effect of climatic factors on the annual variation of the TRW was assessed by a bootstrapped Pearson correlation analysis (Johnson 2001). The tested climatic factors were minimum, maximum, and mean monthly temperature, monthly range of temperature, monthly precipitation, and potential evapotranspiration (PET). Considering the number of tested factors and the length of chronology, a response function analysis was not performed. Local climatic data were obtained from the high-resolution database of the Climatic Research Unit of UEA (Harris et al. 2014) for grid centres located less than 20 km from the studied plantations. Climatic factors were arranged according to the time window from January of the year preceding the formation of tree-ring to September of the year of tree-ring formation. Multicolinearity was assessed for significant factors.
3 Results
Most of the measured time-series of the TRW of hybrid poplar and hybrid aspen were used for the construction of local chronologies (one series for the AUC and KLN and three series for the SKD datasets were rejected). Crossdated time series of TRW showed good agreement (Fig. 1 A, B, C), as the values of EPS exceeded 0.85 and the mean interseries correlation of the datasets was above 0.40 (Table 1). Hybrid poplar displayed a pronounced annual variation of TRW as shown by the mean sensitivity exceeding 0.40. The agreement of TRW of hybrid poplar was better in the AUC than the SKD site, where the environmental signal in the TRW was stronger, as shown by the higher SNR of the time series (10.77 and 6.17, respectively). The agreement and signal strength of the TRW series was even better for the hybrid aspen, as shown by the higher values of interseries correlation, EPS and SNR, although the mean sensitivity was slightly lower (Table 1). The range and mean value of the TRW was higher for hybrid aspens than for poplars, which were older (Table 1); however, at a similar cambial age, the TRW appeared the same (not shown). A decrease in the sample depth was observed after 2010 due to the occurrence of missing rings in a few trees.
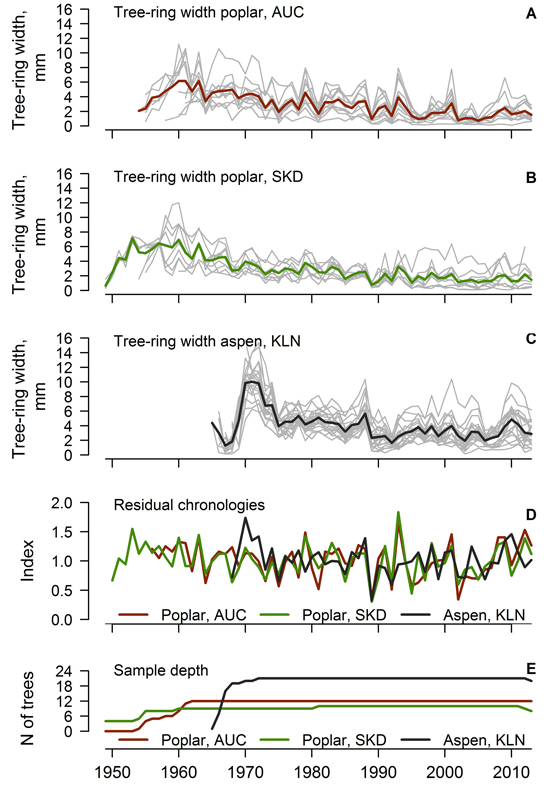
Fig. 1. Crossdated time series of tree-ring width of hybrid poplar in Auce (AUC) (A) and Šķēde (SKD) (B) sites in the western part of Latvia (thick lines represent mean values), crossdated time series of hybrid aspen (C) from Kalsnava (KLN) site in the eastern part of Latvia, residual chronologies of tree-ring width (D) and sample depth of the datasets (E).
| Table 1. Statistics of crossdated datasets of tree-ring width (TRW) of hybrid poplar and hybrid aspen in stands near Auce (AUC), Šķēde (SKD) and Kalsnava (KLN). | |||
| Hybrid poplar | Hybrid aspen | ||
| AUC | SKD | KLN | |
| Covered period | 1954–2013 | 1949–2013 | 1965–2013 |
| Number of crossdated trees | 12 | 10 | 21 |
| Min. TRW, mm | 0.12 | 0.09 | 0.14 |
| Max. TRW, mm | 11.19 | 12.01 | 15.74 |
| Mean TRW, mm | 2.88 | 2.86 | 4.01 |
| Standard deviation, mm | 1.86 | 0.92 | 2.28 |
| Mean sensitivity | 0.42 | 0.40 | 0.35 |
| Mean interseries correlation | 0.51 | 0.42 | 0.51 |
| Autocorrelation | 0.56 | 0.74 | 0.69 |
| Expressed population signal | 0.92 | 0.86 | 0.95 |
| Gleichläufigkeit | 0.69 | 0.68 | 0.70 |
| Signal to noise ratio | 10.77 | 6.17 | 19.74 |
Residual chronologies of TRW were established for each stand (Fig. 1 D). The ranges of chronology indices of hybrid poplar and aspen were similar, but the hybrid aspen had a slightly lower annual variation of TRW indices. Residual chronologies of hybrid poplar were rather synchronous (Fig. 1 D), as the GLK and correlation coefficients calculated between them were 0.77 and 0.78, respectively. In contrast, the high-frequency variation of the TRW of hybrid aspen differed from poplar as suggested by weak correlations between the chronologies (mean r = 0.24). Nonetheless, the mean GLK index calculated between the chronologies of aspen and poplar was 0.51, suggesting similar tendencies in the growth of both hybrids. A few common abrupt changes (decrease) in the TRW occurred in 1975, 1989 and 2002 for both aspen and poplar (Fig. 1 D). These decreases of TRW coincided with extremely high mean monthly temperatures in the December–August period from 1974–1975, 1988–1989 and 2001–2002, accompanied by a rapid drop of temperature in the autumn or winter preceding the formation of the tree-ring.
Residual chronologies of TRW of hybrid poplar and hybrid aspen significantly correlated with 12 and four of the 132 tested factors, respectively, but the values of correlation coefficients did not exceed 0.35. In the case of the hybrid poplar, the sets of the significant correlations were similar at both sites, although some local specifics were apparent (Fig. 2 A, B). Generally, the TRW of hybrid poplar was sensitive to the temperature regime in late summer in the year of tree-ring formation and in the preceding year. A significant effect of PET in August was observed. Precipitation in January of the previous year was the only factor that correlated positively with the TRW of hybrid poplar at both sites. Nevertheless, in the SKD site, the TRW of the hybrid poplar was additionally sensitive to temperature in February, March and June, while the monthly range of temperature in September of the current year was significant at the AUC site. Although the number of significant relationships was lower, the TRW of hybrid aspen generally correlated with the same climatic factors as the hybrid poplar (Fig. 2 C). The mean temperature in March and the temperature range in July and September of the current year had a negative effect on the TRW. Additionally, hybrid aspen showed sensitivity to precipitation in July of the current year, but the value of the correlation coefficient was lower than that observed for other factors.
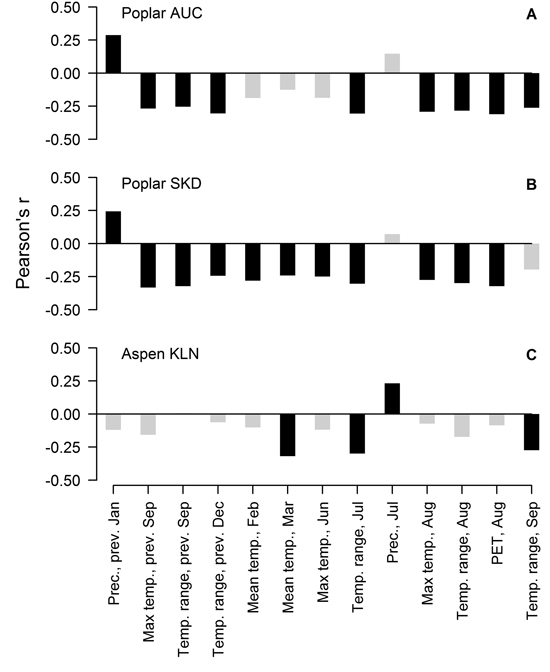
Fig. 2. Significant Pearson correlation coefficients (black bars) between climatic factors and residual chronologies of the TRW of hybrid poplar in Auce (AUC) (A) and Šķēde (SKD) (B) sites in the western part of Latvia and the residual chronology of hybrid aspen at the Kalsnava (KLN) site in the eastern part of Latvia (C). Collinear factors have been omitted. PET-potential evapotranspiration.
4 Discussion
Good agreement and similarity of the high-frequency variation of the time series of the TRW among trees within each stand (Fig. 1 A, B, C) suggested a pronounced effect of common environmental factors on the radial growth of hybrid aspen and hybrid poplar. The EPS values, which exceeded 0.85 (Table 1), showed that the obtained datasets of the TRW were sufficient for the assessment of the local variability of growth (Wigley et al. 1984). The mean sensitivity of the trees was rather high (> 0.35), and the autocorrelation was intermediate (ca. 0.66), suggesting that trees have been promptly reacting to environmental changes (Speer 2010). The SNR of TRW datasets was high (> 6, Table 1), which might be explained by the similar growing conditions (plantation) and by the genetic similarity of the planted material within each of the plantations. The contrast in SNR between the poplar stands (Table 1) likely rose from the difference in climate due to the distance from the sea, which was higher at the AUC site than at the SKD site, resulting in slightly stronger continentality. This is also supported by an even higher SNR (> 19) observed for hybrid aspen (Table 1) in the eastern part of Latvia, where the climate is harsher.
The established local chronologies of hybrid poplar (Fig. 1 D) were similar (r > 0.75), as the same hybrids had been planted, although the sites were located at a ca. 90 km distance away. This suggested that the growth has been affected by common regional-scale factors. The distinction of the high-frequency variation of the TRW of hybrid aspen from hybrid poplar (Fig. 1 D) likely occurred due to the different parental species and their origins. The studied aspens were a hybrid of a northern species, while the parents of the poplars were southern species (Tullus et al. 2013), which are adapted to different conditions (Stettler 1996) and therefore differ in their sensitivity to weather conditions. The differences in the high-frequency variation between aspen and poplar (Fig. 1 D) also might be related to the geographic distance between the sites (ca. 200 km) and to the differences in climate (Fritts 2001) or site conditions. Nevertheless, the occurrence of common decreases in the TRW in 1975, 1989 and 2002 (Fig. 1 D) suggested that both hybrids exhibited similar reaction to weather anomalies. The sensitivity to some common factors has been also supported by the intermediate synchrony between the chronologies of the studied hybrids.
Radial growth of the studied hybrids was sensitive to weather conditions as shown by the significant correlations, but the effect of the tested climatic factors was not strictly limiting for the TRW, as the coefficient values were low (Fig. 2). Considering the higher number of significant factors (Fig. 2), hybrid poplar appeared more sensitive to climate than hybrid aspen although the SNR in the TRW of poplar was lower (Table 1). The weaker signal has apparently been caused by interference among more numerous influencing factors and/or by a shift of their effects over time (Cook 1992; Büntgen et al. 2006). This might be also related to the differences in the high-frequency variation of the TRW between poplar and aspen (Fig. 1 D), although the significant factors for TRW have generally been similar (Fig. 2).
The TRW of hybrid poplar correlated with climatic factors in the current and previous years (Fig. 2 A, B), suggesting a dual effect of weather conditions on the radial increment. The relationships between the TRW and weather conditions in the year preceding the formation of the tree-ring might be explained by the amount of nutrient reserves (Barbaroux and Breda 2002; Pallardy 2008) that accumulate at the end of the growing period and are deployed for early growth in the next spring (Landhäusser and Lieffers 2003; Jones et al. 2004; Regier et al. 2010). The effect of weather conditions of the current year on the TRW might be related to the altered assimilation of nutrients (Berry and Downton 1982), influencing xylogenesis later in the growing period (Miina 2000; Lebourgeois et al. 2005).
Late summer is the period when nutrient reserves are produced (Barbaroux and Breda 2002; Landhäusser and Lieffers 2003; Regier et al. 2010). Increased temperature intensifies evapotranspiration (Traykovic 2005), which might cause a temporary water deficit, resulting in drought stress (Pallardy 2008) and hindering the assimilation (Regier et al. 2009), which would explain the observed negative correlations of the TRW with temperature and PET (Fig. 2 A, B). The hot summers are also usually dry, thus supporting the observed relationships. Additionally, stands of fast growing broadleaved trees are known for intense evapotranspiration, performing as natural pumps (Perry et al. 2001), thus magnifying the effect of a water deficit. Increased summer temperature can also directly hinder cambial activity (xylogenesis) (Deslauriers et al. 2007; Oberhuber and Gruber 2010) and/or photosynthesis (Haldimann and Feller 2004). Formation of latewood, which occurs around August (Deslauriers et al. 2009), is influenced by the current assimilation (Jones et al. 2004), explaining the negative effect of temperature factors of the current summer (Fig. 2). Populus hybrids have a longer growing period compared to their parental species (Yu et al. 2001; Tullus et al. 2011); therefore, the TRW has been sensitive to conditions (temperature) in September (Fig. 2 B). The negative effect of the monthly temperature range during the growing period (Fig. 2) might be related to the stress caused by the shifting environment, as biochemical and physiological processes in trees need to be adjusted to certain conditions (Berry and Downton 1982; Pallardy 2008). September is the time when the first frosts usually occur, particularly when high-pressure systems determine weather; therefore, correlation of the TRW with the temperature range in September (Fig. 2) might be related to a frost damage. The negative effect of temperature and its shifts in the dormant period (Fig. 2) might be explained by cold dehardening in response to thaws (Alden and Herman 1971; Cox and Stushnoff 2001), thus subjecting trees to stronger cold damage in a following drop of temperature (Hänninen 2006). Increased winter temperature can also burden dormancy and cause a depletion of nutrient reserves due to respiration (Foote and Scheadle 1976; Ögren et al. 1997). Precipitation in January is usually in the form of snow. A thicker snow layer has better insulating properties that decrease the depth of soil freeze and minimise fluctuation of soil temperature (Hardy et al. 2001), decreasing root mortality (Tierney et al. 2001) and facilitate water uptake, thus promoting growth (Fig 2, A, B). The one-year lag in reaction of the TRW to winter precipitation (Fig. 2 A, B) might be explained by the allocation of the spared resources to stem growth in the following year (Nguyen et al. 1990; Jones et al. 2004).
The radial increment of hybrid aspen was more affected by the current assimilation than that of poplar as the TRW showed significant correlations with climatic factors in the year of the tree-ring formation (Fig. 2 C). Nevertheless, temperature in March had the strongest effect on the TRW, likely increasing the respiratory loss of nutrient reserves before bud swelling due to burdened dormancy (Foote and Scheadle 1976; Ögren et al. 1997) or increasing the susceptibility to frost damage (Alder and Herman 1971). The negative correlation with July temperature (Fig. 2 C) implied the effect of a water deficit, as observed for the hybrid poplar. However, the hybrid aspen showed a positive reaction to precipitation in the summer (Fig. 2 C), suggesting that it has been able to recover from drought stress more quickly than the poplar and utilise available precipitation for growth as observed for other hybrids (Mazzoleni and Dickmann 1988).
In general, the strength of the observed climate-TRW correlations and number of significant factors for the studied Populus hybrids (Fig. 2), the native pedunculate oak (Quercus robur L.) (Matisons and Brūmelis 2012) and Scots pine (Pinus sylvestris L.) (Elferts 2008) were comparable. Populus hybrids and oak were both sensitive to factors related to water stress in the summer. In contrast, oak and pine showed positive correlations with temperature in the winter months, suggesting that the warming of the climate might be less favourable for the studied hybrids, which have been selected to fit past climates.
5 Conclusions
The growth of the studied Populus hybrids has been significantly affected by weather conditions; however, the observed correlations suggest that none of the tested factors have been strictly limiting. Climatic factors, which are related to water deficit and to drought stress in the summer, have been the main climatic determinants of the TRW, likely via influence on nutrient reserves, current assimilation and hence xylogenesis. Nevertheless, hybrid poplar appeared more affected by the stored nutrient reserves than hybrid aspen. Both of the studied hybrids showed sensitivity to temperature in the winter; however, the correlations were negative, implying that increased temperature burdened the formation of the tree-ring, likely facilitating respiratory loss of nutrient reserves and/or decreasing cold hardiness and subjecting the trees to damage from low temperature. Although the strength of relationships between the TRW and climatic factors was similar, hybrid poplar appeared more sensitive to climatic factors than hybrid aspen, as suggested by the higher number of significant climatic factors. Nevertheless, the observed relationships show that in a warming climate, the radial growth of both poplar hybrids might be burdened due to the increasing water deficit and damage by shifting weather in the winter.
Acknowledgements
The study was funded by European Regional Development Fund project “Fast-growing tree plantations: development of methods of establishment and management and assessment of suitability of wood for production of pellets” (No 2DP/2.1.1.1/13/APIA/VIAA/031). We acknowledge Didzis Elferts for help during the arrangement of the climatic data. Andis Adamovičs, Juris Katrevičs and Juris Kalniņš helped during the sampling and sample preparation. We acknowledge Linda Robalte for the accurate and precise measurements of tree-rings.
References
Alden J., Herman R.K. (1971). Aspects of the cold-hardiness mechanism in plants. The Botanical Review 37: 37–142. http://dx.doi.org/10.1007/BF02860300.
Avotniece Z., Rodinov V., Lizuma L., Briede A., Kļaviņš M. (2010). Trends in frequency of extreme climate events in Latvia. Baltica 23: 135–148.
Barbaroux C., Breda N. (2002). Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse porous beech trees. Tree Physiology 22: 1201–1210. http://dx.doi.org/10.1093/treephys/22.17.1201.
Berry J.A., Downton W.J.S. (1982). Environmental regulation of photosynthesis. In: Govindjee (ed.). Photosynthesis: development, carbon metabolism and plant productivity, Vol. 2. Academic Press, New York. p. 265–345.
Bunn A.G. (2008). A dendrochronology program library in R (dplR). Dendrochronologia 26: 115–124. http://dx.doi.org/10.1016/j.dendro.2008.01.002.
Büntgen U., Frank D.C., Schmidhalter M., Neuwirth B., Seifert M., Esper J. (2006). Growth/climate response shift in a long subalpine spruce chronology. Trees 20: 99–110. http://dx.doi.org/10.1007/s00468-005-0017-3.
Burton L. (2012). Introduction to forestry science, 3rd edition. Cengage Learning, New York. 554 p.
Christersson L. (2007). Poplar plantations for paper and energy in the south of Sweden. Biomass and Bioenergy 32: 997–1000. http://dx.doi.org/10.1016/j.biombioe.2007.12.018.
Cook E.R. (1992). A conceptual linear aggregate model for tree rings. In: Cook E.R., Kairiukstis L.A. (eds.). Methods of dendrochronology: application in the environmental sciences. Kluwer Academic Publishers, Dordrecht. p. 98–104.
Cook E.R., Holmes R.L. (1986). Guide for computer program ARSTAN. In: Holmes R.L., Adams R.K., Fritts H.C. (eds.). Tree-ring chronologies of Western North America: California, eastern Oregon and northern Great Basin. University of Arizona Press, Tucson, Arizona. p. 50–65.
Cox S.E., Stushnoff C. (2001). Temperature-related shifts in soluble carbohydrate content during dormancy and cold acclimation in Populus tremuloides. Canadian Journal of Forest Research 34: 730–737. http://dx.doi.org/10.1139/x00-206.
Čufar K., Prislan P., de Luis M., Gričar J. (2008). Tree-ring variation, wood formation and phenology of beech (Fagus sylvatica) from a representative site in Slovenia, SE Central Europe. Trees 22: 749–758. http://dx.doi.org/10.1007/s00468-008-0235-6.
Deslauriers A., Anfodillo T., Rossi S., Carraro V. (2007). Using simple causal modeling to understand how water and temperature affect daily stem radial variation in trees. Tree Physiology 27: 1125–1136. http://dx.doi.org/10.1093/treephys/27.8.1125.
Deslauriers A., Giovannelli A., Rossi S., Castro G., Frangelli G., Traversi L. (2009). Intra-annual cambial activity and carbon availability in stem of poplar. Tree Physiology 29: 1223–1235. http://dx.doi.org/10.1093/treephys/tpp061.
Elferts D. (2008). Influence of climatic factors on the radial growths of Scots pine Pinus sylvestris L. in Western Latvia on dry soils. University of Latvia, doctoral thesis. 84 p.
Foote K.C., Scheadle M. (1976). Diurnal and seasonal patterns of photosynthesis and respiration by stems of Populus tremuloides Michx. Plant Physiology 58: 651–655. http://dx.doi.org/10.1104/pp.58.5.651.
Fritts H.C. (2001). Tree-rings and Climate. The Blackburn Press, Caldwell. 582 p.
Grissino-Mayer H.D. (2001). Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree-Ring Research 57: 205–221.
Haldimann P., Feller U. (2004). Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell and Environment 27: 1169–1183. http://dx.doi.org/10.1111/j.1365-3040.2004.01222.x.
Hänninen H., 2006. Climate warming and the risk of frost damage to boreal forest trees: identification of critical ecophysiological traits. Tree Physiology 26: 889 –898. http://dx.doi.org/10.1093/treephys/26.7.889.
Hardy J.P., Groffman P.M., Fitzhugh R.D., Henry K.S., Welman A.T., Demers J.D., Fahey T.J., Driscoll C.T., Tierney G.L., Nolan S. (2001). Snow depth manipulation and its influence on soil frost and water dynamics in a northern hardwood forest. Biogeochemistry 56: 151–174. http://dx.doi.org/10.1023/A:1013036803050.
Harris I., Jones P.D., Osborn T.J., Lister D.H. (2014). Updated high-resolution grids of monthly climatic observations – the CRU TS3.10 Dataset. International Journal of Climatology 34: 623–642. http://dx.doi.org/10.1002/joc.3711.
Hughes M.K., Funkhouser G. (2003). Frequency-dependent climate signal in upper and lower forest border tree rings in the mountains of the Great Basin. In: Diaz H.F. (ed.). Climate variability and change in high elevation regions: past, present and future. Springer, New York. p. 233–244. http://dx.doi.org/10.1007/978-94-015-1252-7_11.
Jansons Ā., Zeps M., Rieksts-Riekstiņš J., Matisons R., Krišāns O. (2014). Height increment of hybrid aspen Populus tremuloides × P. tremula as a function of weather conditions in southwestern part of Latvia. Silva Fennica vol. 48 no. 5 article 1124. 13 p. http://dx.doi.org/10.14214/sf.1124.
Johnson R.W. (2001). An introduction to bootstrap. Teaching Statistics 23: 49–54. http://dx.doi.org/10.1111/1467-9639.00050.
Jones B., Tardif J., Westwood R. (2004). Weekly xylem production in trembling aspen (Populus tremuloides) in response to artificial defoliation. Canadian Journal of Botany 82: 590 –597. http://dx.doi.org/10.1139/b04-032.
Kirschbaum M.U.F. (2000). Forest growth and species distribution in a changing climate. Tree Physiology 20: 309–322. http://dx.doi.org/10.1093/treephys/20.5-6.309.
Klavins M., Rodinov V. (2010). Influence of large-scale atmospheric circulation on climate in Latvia. Boreal Environment Research 15: 533–543.
Landhäusser S.M., Lieffers V.J. (2003). Seasonal changes in carbohydrate reserves in mature northern Populus tremuloides clones. Trees 17: 471–476. http://dx.doi.org/10.1007/s00468-003-0263-1.
Lebourgeois F., Breda N., Ulrich E., Granier A. (2005). Climate-tree-growth relationships of European beech (Fagus sylvatica L.) in the French Permanent Plot Network (RENECOFOR). Trees 19: 385–401. http://dx.doi.org/10.1007/s00468-004-0397-9.
Lindner M., Maroschek M., Netherer S., Kremer A., Barbati A., Garcia-Gonzalo J., Seidl R., Delzon S., Corona P., Kolström M., Lexer M.J., Marchetti M. (2010). Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. Forest Ecology and Management 259: 698–709. http://dx.doi.org10.1016/j.foreco.2009.09.023.
Lizuma L., Kļaviņš M., Briede A., Rodinovs V. (2007). Long-term changes of air temperature in Latvia. In: Kļaviņš M. (ed.). Climate change in Latvia. University of Latvia, Riga, Latvia. p. 11–20.
Mangalis I. (2004). Regeneration and establishment of forest. [Meža atjaunošana un ieaudzēšana]. Et Cetera, Riga. 455 p [In Latvian].
Matisons R., Brūmelis G (2012). Influence of climate on tree-ring and earlywood vessel formation in Quercus robur in Latvia. Trees-Structure and Function 26: 1251–1266. http://dx.doi.org/ 10.1007/s00468-012-0701-z.
Mazzoleni S., Dickmann D.I. (1988). Differential physiological and morphological responses of two hybrid Populus clones to water stress. Tree Physiology 4: 61 –70. http://dx.doi.org/10.1093/treephys/4.1.61.
Miina J. (2000). Dependence of tree-ring, earlywood and latewood indices of Scots pine and Norway spruce on climatic factors in eastern Finland. Ecological Modelling 132: 259–273. http://dx.doi.org/10.1016/S0304-3800(00)00296-9.
Nguyen P.V., Dickmann D.I., Pregitzer K.S., Hendrick R. (1990). Late-season changes in allocation of starch and sugar to shoots, coarse roots, and fine roots in two hybrid poplar clones. Tree Physiology 7: 95–105. http://dx.doi.org/10.1093/treephys/7.1-2-3-4.95.
Oberhuber W., Gruber A. (2010). Climatic influences on intra-annual stem radial increment of Pinus sylvestris (L.) exposed to drought. Trees 24: 887–898. http://dx.doi.org/10.1007/s00468-010-0458-1.
Ögren E., Nilsson T., Sundblad L.G. (1997). Relationship between respiratory depletion of sugars and loss of cold hardiness in coniferous seedlings over-wintering at raised temperatures: indications of different sensitivities of spruce and pine. Plant, Cell and Environment 20: 47–253. http://dx.doi.org/10.1046/j.1365-3040.1997.d01-56.x.
Pallardy S.G. (2008). Physiology of woody plants, third ed. Elsevier, London, UK. 464 p.
Perry C.H., Miller R.C., Brooks K.N. (2001). Impacts of short-rotation hybrid poplar plantations on regional water yield. Forest Ecology and Management 143: 143 –151. http://dx.doi.org/10.1016/S0378-1127(00)00513-2.
Regier N., Streb S., Cocozza C., Schaub M., Cherubini P., Zeeman S.C., Frey B. (2009). Drought tolerance of two black poplar (Populus nigra L.) clones: contribution of carbohydrates and oxidative stress defence. Plant, Cell and Environment 32: 1724 –1736. http://dx.doi.org/10.1111/j.1365-3040.2009.02030.x.
Regier N., Streb S., Zeeman S.C., Frey B. (2010). Seasonal changes in starch and sugar content of poplar (Populus deltoides × nigra cv. Dorskamp) and the impact of stem girdling on carbohydrate allocation to roots. Tree Physiology 30: 979–987. http://dx.doi.org/10.1093/treephys/tpq047.
Schueler V., Weddige U., Beringer T., Gamba L., Lamers P. (2013). Global biomass potentials under sustainability restrictions defined by the European Renewable Energy Directive 2009/28/ EC. GCB Bioenergy 5: 652–663. http://dx.doi.org/10.1111/gcbb.12036.
Seo J.W., Eckstein D., Jalkanen R., Schmitt U. (2011). Climatic control of intra- and inter-annual wood-formation dynamics of Scots pine in northern Finland. Environmental and Experimental Botany 72: 422–431. http://dx.doi.org/10.1016/j.envexpbot.2011.01.003.
Speer J.H. (2010). Fundamentals of tree-ring research. The University of Arizona Press, Tucson, Arizona. 333 p.
Stettler R.F. (1996). Biology of Populus and its implications for management and conservation. NRC Research Press, Ottawa. 539 p. http://dx.doi.org/10.1139/cjm-2014-0362.
Tierney G.L., Fahey T.J., Groffman P.M., Hardy J.P., Fitzhugh R.D., Driscoll C.T. (2001). Soil freezing alters fine root dynamics in a northern hardwood forest. Biogeochemistry 56: 175–190. http://dx.doi.org/10.1023/A:1013072519889.
Traykovic S. (2005). Temperature-based approaches for estimating reference evapotranspiration. Journal of Irrigation and Drainage Engineering-ASCE 131: 316–323. http://dx.doi.org/10.1061/(ASCE)0733-9437(2005)131:4(316).
Tullus A., Rytter L., Tullus T., Weih M., Tullus H. (2011). Short-rotation forestry with hybrid aspen (Populus tremula L. × P. tremuloides Michx.) in Northern Europe. Scandinavian Journal of Forest Research 27: 10–29. http://dx.doi.org/10.1080/02827581.2011.628949.
Tullus H., Tullus A., Rytter L. (2013). Short-rotation forestry for supplying biomass for energy production. In: Kellomäki S., Kilpeläinen A., Alam A. (eds.). Forest bioenergy production. Springer, New York. p. 39–56. http://dx.doi.org/10.1007/978-1-4614-8391-5_3.
Wigley T.M.L., Briffa K.R., Jones P.D. (1984). On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. Journal of Climate and Applied Meteorology 23: 201–213. http://dx.doi.org/10.1175/1520-0450(1984)023<0201:OTAVOC>2.0.CO;2.
Yu Q., Tigerstedt P.M.A., Haapanen M. (2001). Growth and phenology of hybrid aspen clones (Populus tremula L. × Populus tremuloides Michx.). Silva Fennica 35: 15–25. http://dx.doi.org/10.14214/sf.600.
Total of 53 references.

