The effect of stem girdling on xylem and phloem formation in Scots pine
Fajstavr M., Giagli K., Vavrčík H., Gryc V., Urban J. (2017). The effect of stem girdling on xylem and phloem formation in Scots pine. Silva Fennica vol. 51 no. 4 article id 1760. https://doi.org/10.14214/sf.1760
Highlights
- Stem girdling ceased the cambial activity, below the girdled area, immediately after the removal of the bark strip
- Pinus sylvestris survived for up to two years after stem girdling
- The girdled trees formed phloem cells above the girdled area but failed to form latewood cells in the next growing season.
Abstract
Scots pine (Pinus sylvestris L.) is a resilient, wide spread species. This paper reports on the xylem and phloem cell formation process, before and after, the species was put under artificial stress by stem girdling. Microcore method was applied to a healthy control group and a standing group of girdled trees within an 80-year-old pine forest for two consecutive growing seasons (2013 and 2014). The stem girdling was applied in the middle of the first growing season (July 2013). Cambial activity timings (onset and cessation of cell division), cell formation intensity, cell differentiation, and the dynamics of the annual radial increment in the stem were analyzed. Cambial activity was inhibited and eventually ceased below the stem girdling immediately after the removal of the strip. Therefore, no latewood tracheids were formed. However, above the stem girdling and in the control trees, cell formation and tissue differentiation continued until the end of the growing season, with the girdled trees moving at a less intensive pace but for a longer period of time. During the following growing season (2014), the cambial zone was reactivated only above the stem girdling, not below, and eventually the girdled trees died. In 2014, the onset of the cambial activity was delayed and the division rate of the cells was slower in the girdled trees. Furthermore, the girdled trees formed less phloem cells than the control trees.
Keywords
Pinus sylvestris;
radial increment;
cambial activity;
cell differentiation;
phloemogenesis;
xylogenesis
-
Fajstavr,
Department of Wood Science, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelska 3, 613 00 Brno, Czech Republic
E-mail
fajstavr.marek@seznam.cz

- Giagli, Department of Wood Science, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelska 3, 613 00 Brno, Czech Republic E-mail kyriaki.giagli@mendelu.cz
- Vavrčík, Department of Wood Science, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelska 3, 613 00 Brno, Czech Republic E-mail vavrcik@mendelu.cz
- Gryc, Department of Wood Science, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelska 3, 613 00 Brno, Czech Republic E-mail gryc@mendelu.cz
- Urban, Department of Forest Botany, Dendrology and Geobiocenology, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelska 3, 613 00 Brno, Czech Republic; Siberian Federal University, Svobodnyj Prospect 79, Krasnoyarsk, 660041 Krasnoyarsk, Russia E-mail josef.urban@email.cz
Received 19 December 2016 Accepted 18 July 2017 Published 22 August 2017
Views 202387
Available at https://doi.org/10.14214/sf.1760 | Download PDF

1 Introduction
Scots pine (Pinus sylvestris L.) has a high level of ecological tolerance, and is strongly resistant against the influence of extreme climate and other stress factors (Kelly and Connolly 2000). The species is the most widely distributed pine species on the planet (Bogino et al. 2009), it grows in a wide variety of areas and in all altitude zones (Gruber et al. 2010). In Central Europe, it is dominant on extreme sites in open forests, i.e. rocky areas with big outcrops of various bedrock, peaty soils or other nutrient-poor habitats (Richardson and Rundel 1998). In the Czech Republic, Scots pine forests mixed with a low but regular occurrence of Norway spruce, oaks and shrubs (Vaccinium spp.) have prevailed since the late Boreal period, with an insignificant change in vegetation, except for succession after fire events (Novák et al. 2012). Pinewoods cover approximately 17% of the total timber land, the second most widespread after the Norway spruce (Ministry report 2014), taking up a significant position in all vegetation zonas (Chytrý et al. 2010; Vacek et al. 2016).
According to Rossi et al. (2012), the relationships between the phenological phases, generating the complex pattern of cells during wood formation, still remain essentially unkown.
Nevertheless, several studies on wood formation in Scots pine have been conducted all over Europe. The key enviromental factors infuencing the secondary wood growth of the species have been the focus of numerous studies. Wodzicki (1971) described the complexity of the xylem differentiation process of Scots pine in Poland. In Austria, Scots pine trees growing on xeric and dry-mesic sites were examined (Gruber et al. 2010; Oberhuber et al. 2011; Swidrak et al. 2011, 2014), in Finland (Schmitt et al. 2004; Seo et al. 2011; Jyske et al. 2014), in Siberia (Antonova and Stasova 1993; Antonova et al. 1995), in France (Rathgeber et al. 2011; Cuny et al. 2012, 2014) and in Spain (Martinez del Castillo et al. 2016). Rossi et al. (2013) and Cuny et al. (2015), included Scots pine, among several conifers, in a compilation of data on cambium phenology and wood-formation dynamics. These studies detected the complex relationship between the plasticity of tree-ring formation and the enviromental factors, focussing mostly upon the stress caused by extreme terrains or weather events. To draw conclusions on the plasticity of the differentiation process during xylem and phloem formation, we decided to induce an extreme, artificial stress factor to the Scots pine, by applying stem girdling.
Stem girdling consists of the removal of an entire strip of bark down to the vascular cambium, blocking the phloem flow of photosynthates from reaching the root system and/or other parts of the plant (Jordan and Habib 1996; Tombesi et al. 2014). Traditionally, girdling has been applied for apical control of branch growth in studies (Münch 1938). Stem girdling, as one of the most practical methods to provoke artificial stress (Stone 1974; Wilson 1998), was broadly used over the decades in horticulture to manage the growth and reproduction of several deciduous and evergreen tree species (Weinburger and Cullinan 1932; Lilleland and Brown 1936; Crane and Campbell 1957; Lewis and McCarty 1973; Winkler et al. 1974; Powell and Cash Howell 1985; Fernandez-Escobar et al. 1987; Augusti et al. 1998; Day and DeJong 1999; Rivas et al. 2008). Girdling has been practiced in the forestry and logging industry as a pre-harvest treatment to change wood properties (MacDougal 1943; Noel 1970; Lopez et al. 2015) by manipulating annual ring width, wood density, duration of cambial activity, and latewood production (Wilson and Gartner 2002; Domec and Pruyn 2008; Maunoury-Danger et al. 2010; Sellin et al. 2013). More recently, several researchers have applied girdling for physiological experiments primarily to manipulate carbohydrate distribution within plants (De Schepper and Steppe 2011) and to study phloem–xylem interactions (Fishman et al. 2001; Wilson and Gartner 2002; Zwieniecki et al. 2004; Salleo et al. 2006; Morandi et al. 2007; Domec and Pruyn 2008; Tombesi et al. 2014).
The girdled trees usually suffer a significant reduction in water content, especially in the area below the girdling (Noel 1970; Taylor 1999), decreasing the hydraulic conductivity (Salleo et al. 1996; Zwieniecki and Holbrook 1998; Zwieniecki 2000). Noel (1970) and Taylor (1999) confirmed that below the girdled area the content of starch and other carbohydrates decreased, having a direct impact on the xylem and phloem growth. In contrast, the cambial activity above the girdling was usually only temporarily paused, if at all (Noel 1970; Barbosa and Wagner 1989). In some cases, a small amount of nutrients can still be supplied to the roots of the girdled trees from the above ground part of the tree by ray parenchyma, that bridges the girdled strip, or from neighbours by root grafts. In such cases, roots, together with the below girdling area may continue to grow. Recently, Lopez et al. (2015) underlined that girdling influenced both the downward carbon flow and the upward water flow by blocking the root sink. Hence, below girdling, a noticeable decrease of radial growth was recorded, accompanied by profuse sprouting which stopped after the depletion of root stored carbohydrates. Noel (1970) reported that cambial activity below the girdling was limited in a severe and permanent way, producing deficient growth rings, while the radial increment above the girdling increased (Lopez et al. 2015). However, little is known about the impact of girdling on the secondary phloem and secondary xylem cells which are produced by the cambium (Larson 1994; Pang et al. 2008).
Many trees have survived for a considerable period after girdling, even without healing. Several species of North American, European and African trees remained alive for 1 to 5 years after girdling, with the length of survival varying among tree species. Pine and larch trees seem to be more resistant than the others e.g., resinous pine (Pinus resinosa L.) and American larch (Larix laricina (Du Roi) K. Koch) remained alive for 1–2 years after girdling (Taylor 1999; Wilson and Gartner 2002). In contrast, Norway spruce (Picea abies (L.) H. Karst.) and red spruce (Picea rubens Sarg.) trees died immediately after the girdling (Stone 1974). Wagener (1961) suggested that white pine (Pinus strobus L.) is able to survive girdling of up to 60% of their circumference while other conifer species have a low chance of survival if more than 25% of their circumference is girdled at the base. Ryan (1990) reported that in the absence of a significant crown injury, most trees survived up to 25% basal girdling, few trees survived more than 75% girdling, and approximately 50% of trees survived within this range (Schmitt and Filip 2005).
Girdling woody stems may have traumatic effects in addition to releasing branches from apical control. These effects vary widely among species (Noel 1970). Wilson (1968) applied the girdling to white pine by removing a bark strip of 2 cm. The meristematic function and the secondary cell growth was inhibited, and eventually stopped, beneath the girdled area. The cells formed before girdling, completed the differentiation stage during the remaining days of the growing season but no new cells were produced (Wilson 1968). Therefore, the length of the survival period after girdling is crucial for monitoring the differences in xylem and phloem formation between the two sides of the girdled stem.
The objective of this study was to determine, with the use of the microcore method, (1) how cambial activity takes places when Scots pine trees undergo serious stress, induced by stem girdling (2) how xylem and phloem formation progresses when girdling stress occurs during the growing season (3) how the phenological phases of xylo- and phloemogenesis differentiated above and below the girdled area, and (4) how long can Scots pine trees survive after total stem girdling.
In order to better understand the duration and the extension of the corresponding anatomical changes, we studied the cambial activity timings and dynamics of xylem-phloem cell formation of girdled Scots pine trees for two consecutive growing seasons (2013 and 2014).
2 Materials and methods
2.1 Site characteristics
The sampling was conducted in the Training Forest Enterprise Masaryk Forest Křtiny research plot (49°15´N, 16°36´E, 404 m a.s.l.) in Soběšice, Czech Republic. The forest consisted of 70% Scots pine mixed with larch and several deciduous species. According to the long-term data (1901–2014), the mean annual air temperature is 8.1 °C and the mean annual precipitation is 601 mm. The warmest month in the area is July (18.1 °C) and the coldest is January (–2.7 °C). July (82 mm) is also the month with the highest amount of precipitation while the driest month is February (27.5 mm) (Climate Research Unit Time Series, CRU TS3.23; via http://climexp.knmi.nl).
Weather data was obtained directly from the research plot during the two examined years (2013 and 2014). We measured air temperature (Minikin TRH, EMS Brno, Czech Republic) and precipitation (MetOne Instruments, Grants Pass, Oregon, USA). Soil water potential was measured directly on the plot at depths of 15 cm, 50 cm and 90 cm in two repetitions (Delmhorst Inc., Towaco, NJ, USA attached to datalogger SP3, EMS Brno, Czech Republic). Data was acquired every 10 mins and stored in a data logger. We calculated the weekly precipitation amount, the average weekly air temperature, and the average soil water potential (based on measurements obtained from all three depths). The sum of the effective temperature was calculated as the sum of the mean daytime temperature for the stated periods. The threshold temperature was 5 °C, values below that were counted as zero.
2.2 Girdling
Twelve dominant healthy 80-year-old Scots pine trees were chosen, the trees were on average, 24 m in height and 33 cm in diameter at breast height (130 cm). In 2013, during the growing period (July 11; DOY 192), six of the twelve selected trees underwent the girdling treatment, i.e. a complete layer of bark and phloem strip (5 cm) was removed around the stem circumference at breast height (130 cm) by chainsaw (Fig. 1) (Daudet et al. 2005; Tombesi at al. 2014; Choi et al. 2010). After the treatment, we studied the six girdled trees and compared them with the six remaining, untreated trees (the control).
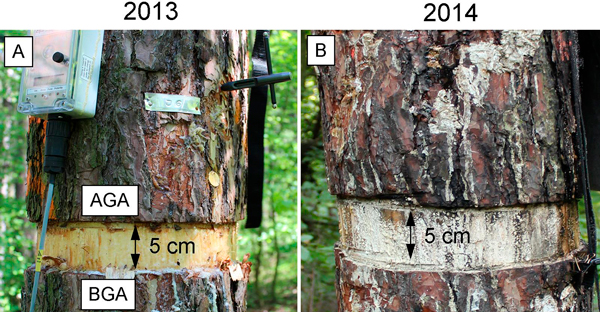
Fig. 1. Microcore sampling of girdled stems during the growing seasons of 2013 and 2014. A – the first week of July 2013 (week after girdling). B – dieback of girdled trees in July 2014. AGA – above girdling area; BGA – below girdling area.
2.3 Sampling
Two microcores, with a thickness of 1.8 mm, were collected from the tree stems at weekly intervals using the Trephor tool (Rossi et al. 2006). The distance between individual microcores, on the stem, was more than 2 cm in order to prevent the damage caused to the surrounding tissue. The sampling started on April 4 (DOY 94, 2013) and ended on November 16 (DOY 320, 2013). The date of the girdling was July 11 (DOY 192, 2013). Hence, the sampling was performed in two phases; before and after the treatment. From April 4 to July 11, two microcores were taken weekly, per tree (12 trees) at breast height. After the treatment, from July 12 to November 16, we took four microcores per tree; two from above (AGA) and two from below the stem (BGA) girdling. The sampling of the control trees remained unchanged. In 2014, the procedure was the same as the after treatment phase in 2013.
2.4 Sample preparation
The microcores were immediately immersed into FAA solution (90 ml of 70% ethanol, 5 ml of acetic acid, 5 ml of 36–38% formaldehyde), for one week, and then washed (water) and stored in 50% ethanol.
The microcores were dehydrated in ethanol series’ (70%, 90%, 95% and 100%) and then infiltrated with paraffin in a tissue processor (Leica TP1020). After the paraffin infiltration (four hours) the microcores were poured, together with histological cases, into paraffin blocks (by Leica EG1120 dispenser) and cut by the rotary microtome (Leica RM2235). The micro cuts were dried in a laboratory oven (70 °C for 20 minutes). Subsequently, the micro cuts were stained by safranin and astra-blue mixture, to distinguish the lignified and non-lignified tissues.
2.5 Measurements and data processing
The cambial cells (CC) comprise the cambial initial cells along with both xylem and phloem mother cells (Plomion et al. 2001). The CC were flattened tangentially, rectangular- or hexagonal-shaped with a very thin cell wall middle lamella (Wodzicki 1971). The onset of cambial activity was defined as when the CC started to be metabolically active, gradually dividing, and hence, increasing in number (Figs. 2A, B) (Prislan et al. 2016).
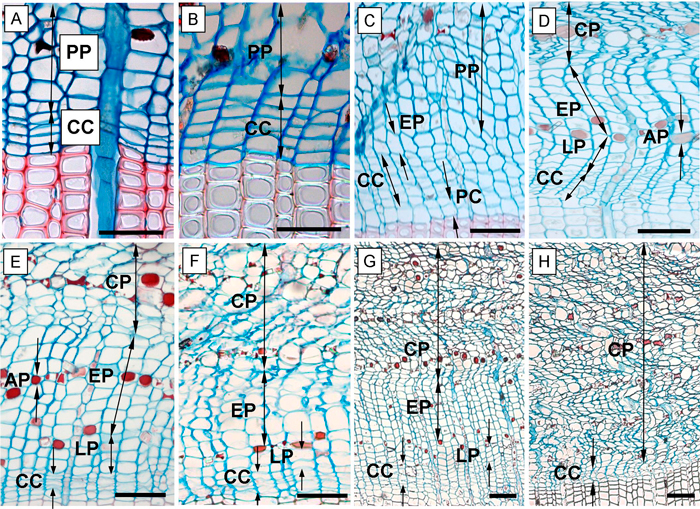
Fig. 2. Phases of annual phloem formation during the growing seasons of 2013 and 2014. A – dormant phase of cambium; B – onset of cambial activity (swelling of cambial cells); C – the first forming row of early phloem and early wood; D – fully formed annual phloem increment; E, F – phloem increment at the end of the 2013 growing season in the above girdling area (E) and below girdling area (F); G – formed annual phloem increment within above area of girdling on June 5, 2014; H – the below area of girdling with non-activated cambium and without cell increment (June 5, 2014). CC – cambial cells; PP – sieve cells of previous annual phloem increment; EP – early phloem; LP – late phloem; AP – axial parenchyma; CP – collapsing phloem cells; PC – phase of post-cambial growth. Scale bars = 100 µm.
We recorded the following phases of xylem cell development: the post-cambial growth (PC), the growth and lignifications of the secondary cell wall (SW), and the mature cells (tracheids) phase (MT) (Romagnoli et al. 2011). In the PC phase, the cells had thin, non-lignified cell walls which were stained blue (astra-blue staining) and showed no glistening under a polarized light (Fig. 3F). This is the phase when the cells started enlarging and elongating.
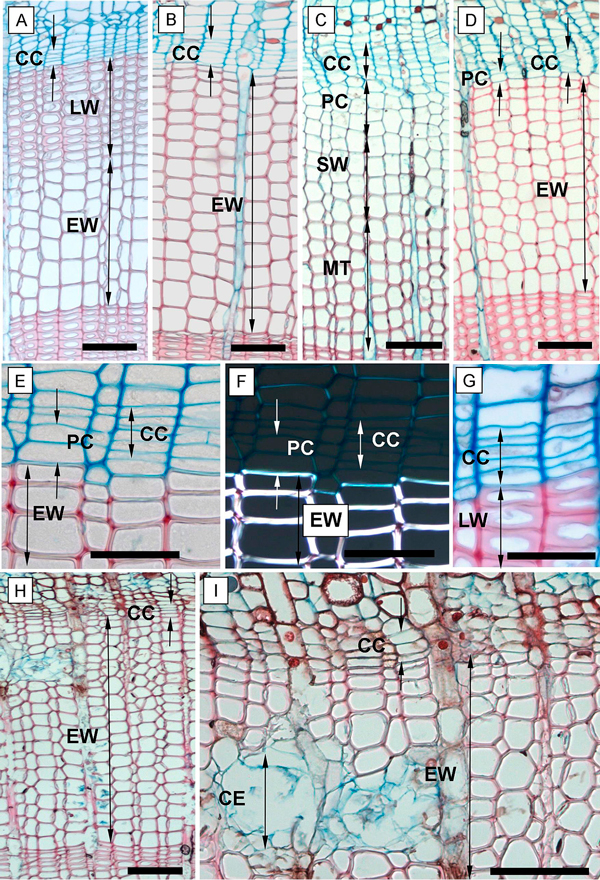
Fig. 3. Phases of xylem formation during the 2013 and 2014 growing seasons. A, B – annual ring at the end of the 2013 growing season in the above girdling area (A) and the below girdling area (B); C – tree ring formation in May 2014 of the above girdling area; D – below girdling area in May 2014 with inactivated cambium and without cell increment; E – tree ring in the below girdling area (November 2013) with undifferentiated cells of PC; F – the undifferentiated cells of PC and fully formed cells of EW under polarized light; G – fully formed and differentiated late tracheids in above girdling area (November 2013); H, I – necrosis inside girdled stems in September 2014. CC – cambial cells; PC – phase of post-cambial growth; SW – phase of secondary wall deposition; MT – completely matured tracheids; EW – earlywood; LW – latewood; CE – collapsing epithelial cells of resin canals. Scale bars: A, B, C, D, H, I = 100 µm; E, F, G = 50 µm.
The following SW phase was distinguished by the cells starting to glisten under the polarized light (Fig. 3F). During this phase, the lignification process also occurred; lignified cell wall tissue responded to the safranin solution and began to gradually replace the blue stain with the red stain. In the MT phase, we recorded fully mature tracheids with completely lignified cell walls (fully red-stained) and a totally empty lumen (depleted cell nucleus) (Fig. 3C).
We also recorded the following phases of phloem cell development: the sieve cells of early phloem (EP) and late phloem (LP) as well as the axial parenchyma (AP) were also recorded. The EP sieve cells were identified due to the larger radial dimensions compared with cambial cells (Fig. 2C). In many coniferous species, the pre-existent phloem cells are compressed by the newly formed ones and hence collapse. Nevertheless, in Scots pine this occurs later. The marginal zone between newly formed and old growth cells is indiscernible but detectable by the increased number of large cells (Fig. 2C). The LP sieve cells were distinguished from EP sieve cells by the presence of the AP cells which formed aborder of lumen filled, red stained, storage cells between them (Fig. 2D). Furthermore, the LP phase contained a notably lower amount of sieve cells compared to the EP sieve cells (Gričar 2007).
The sections were observed and analyzed under a light microscope (Leica DMLS) using a digital camera (Leica DFC 280) and the public domain image processing software ImageJ (Abramoff et al. 2004). Three radial cell files within periodically formed increments were chosen and counted (Deslauriers et al. 2008).
The Gompertz function simulated the length of radial increments during the growing season and defined the growth intensity by calculating the total number of cells formed. The model (1st derivative) determined the daily number of cells formed per year. The data were fitted with a sigma-shaped growth curve (Eq. 1), produced for six control and six girdled (above and below girdling) trees (TableCurve 2D) (Rossi et al. 2003).
![]()
, where y – weekly cumulative number of cells, t – day of the year, A – upper asymptote of the maximum number of cells, β – x-axis placement parameter, κ – inflection point on the curve representing the maximum daily rate of growth.
The statistical analysis evaluated differences between the groups of control and girdled trees above and below the girdling. Since the growing season began before the girdling and samples were taken, all sample trees were evaluated together until July 11 (2013) (n = 12) and then split into three groups with the added below the girdling samples (n = 6 in each of the groups).
The Kruskal–Wallis test (α = 0.05) was used for testing whether samples originated from the same distribution. The test does not assume a normal distribution of the residuals and equal variances, unlike the analogous one-way analysis of variance (ANOVA). The Kruskal–Wallis test was done in R software.
3 Results
3.1 Weather conditions
In 2013, the amount of precipitation was found to be extremly low for a number of weeks during growing season (Fig. 4A). We measured only 2 mm of precipitation during the whole of July (DOY 182–211), which is in contrast to the long-term data, where July is wettest month of the year in this area. The dry period was confirmed by soil water potential findings (Fig. 4C). August (DOY 212–244) showed an increase (40.80 mm) in the amount of precipitation, but this is also considered to be dry, when compared to the long-term data.
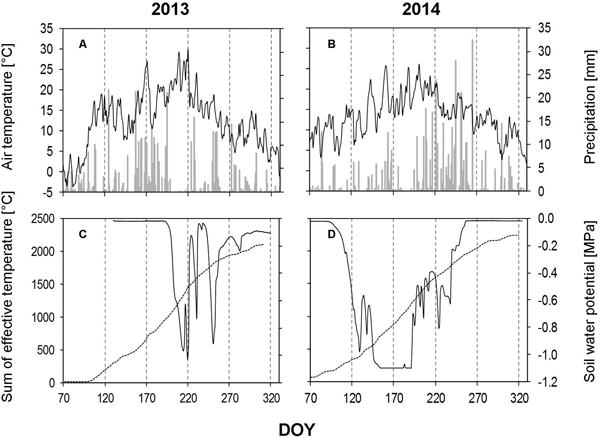
Fig. 4. Weather data and soil water potential recorded during the growing seasons of 2013 and 2014. A, B – daily precipitation amount (bars) and average daily air temperature (solid line); C, D – average daily soil water potential based on measurements obtained from all three depths (solid line) and sum of the effective temperature (dashed line). DOY – day of the year (70: March 11, 120: May 30, 170: June 19, 220: August 8, 270: September 27, 320: November 16).
In 2014, the growing season started with low amounts of precipitaion, during March (17.4 mm), April (20.4) and May (60.4 mm), these levels are almost half of those recorded in 2013 (Fig. 4B). These low levels of precipitation occurred at the beginning of the vegetation period and initiated an early dry period (mid-May), which continued until mid-July. June and July recorded the highest average monthly temperatures of the year (around 22 °C). The low amount of precipitation in combination with the high temperatures was depicted again in the soil water potential (Fig. 4D).
3.2 Growth period during treatment (2013)
3.2.1 Cambial activity
During dormancy, the cambial zone was composed of 5.5 ± 0.6 cells (Fig. 5A). The onset of cambial activity was measured in all sample trees together, since the stem girdling took place later. No significant differences among the trees were found. The cambial reactivation occurred between April 18 and 25, when the cambial zone contained 6.1 ± 0.6 cells and the sum of the effective temperature was 136.1 °C (Fig. 4C). The number of cambial cells gradually increased to 8.8 ± 1.2 by May 9 (DOY 129). The number of cells in the cambial zone was 7.6 ± 1.2 cells when the stem girdling was performed on July 11 (DOY 192).
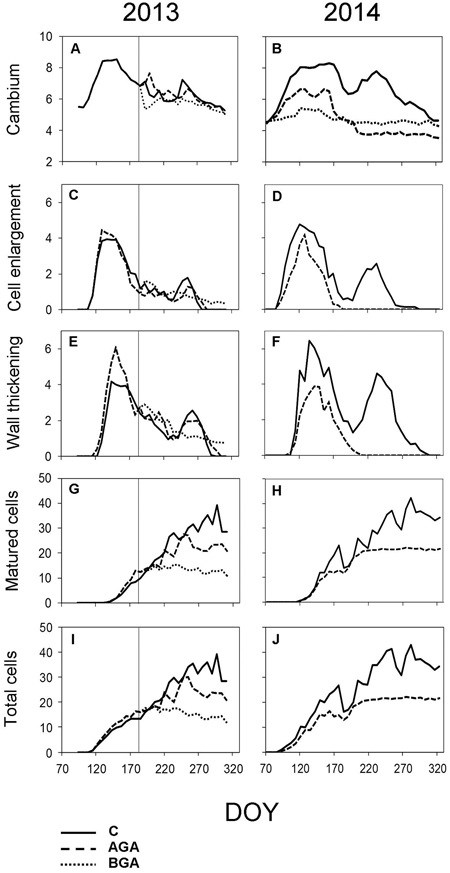
Fig. 5. Dynamics of xylem formation and cambial activity during the 2013 and 2014 growing seasons. Number of cells within cambial zone (A, B), in PC phase (C, D), in SW phase (E, F), in MT phase (G, H) and the total cell numbers within a fully formed annual tree ring (I, J). C – control trees (solid line); AGA – above girdling area (dashed line); BGA – below girdling area (dotted line). DOY – day of the year (70: March 11, 120: May 30, 170: June 19, 220: August 8, 270: September 27, 320: November 16). Solid vertical line – date of girdling.
In the control trees, the cambial activity was noticeably reduced from July to August and was reactivated in September, maintaining 6.5 ± 0.5 cells. The cambial activity of the control trees continued until September 18 (DOY 261). The number of cambial cells by the end of the growing season was almost equal to the number of cambial cells contained before the reactivation of the cambial zone.
No significant difference was found in the timing or duration of cambial activity between the control trees and the above stem girdling group (Table 1). However, the cambial zone above the girdling underwent a latent phase (6.6 ± 0.5 cells) which lasted until the end of August. Subsequently, the cambial activity was reactivated again, the number of cambial cells fluctuated from 6 to 7 until the final cessation of cambial activity on October 1 (DOY 274). Below the stem girdling, the cambial activity was ceased after the treatment.
| Table 1. Cambial activity and differentiation of xylem cells timings in six control and six girdled (above girdling) trees (2013 and 2014). | |||||||
| 2013 | 2014 | ||||||
| Parameters (DOY) | Control | AGA | K–W test | Control | AGA | K–W test | |
| CA | 108 ± 0 | 113 ± 6 | 0.058 | 87 ± 3 | 93 ± 4 | 0.026 | |
| PC | 116 ± 3 | 116 ± 3 | 1 | 94 ± 3 | 97 ± 4 | 0.241 | |
| SW | 133 ± 4 | 130 ± 5 | 0.423 | 115 ± 3 | 119 ± 7 | 0.222 | |
| MT | 148 ± 7 | 147 ± 4 | 0.600 | 127 ± 7 | 127 ± 10 | 1 | |
| CCA | 261 ± 27 | 274 ± 8 | 0.389 | 277 ± 14 | 168 ± 16 | 0.003 | |
| CPC | 259 ± 33 | 264 ± 39 | 0.131 | 268 ± 15 | 174 ± 11 | 0.004 | |
| CSW | 278 ± 29 | 296 ± 14 | 0.029 | 293 ± 13 | 190 ± 31 | 0.004 | |
| CA duration (days) | 153 ± 27 | 161 ± 6 | 1 | 190 ± 14 | 75 ± 18 | 0.004 | |
| PC duration (days) | 144 ± 36 | 148 ± 39 | 0.246 | 174 ± 12 | 77 ± 11 | 0.004 | |
| SW duration (days) | 146 ± 30 | 166 ± 10 | 0.032 | 177 ± 11 | 72 ± 33 | 0.004 | |
| P-values for Kruskal–Wallis test (H0 – Medians of all groups are equal, if p < alpha (0.05) > rejecting H0). DOY – day of year; Control – control trees; AGA – above girdling area; K–W test – results (p-values) of Kruskal–Wallis test; CA – onset of cambial activity; CCA – cessation of CA; PC – onset of postcambial growth (cell enlargement); CPC – cessation of PC; SW – onset of secondary wall deposition; CSW – cessation of SW; MT – occurrence of the first matured tracheids. Bold – statistically significant difference (alpha = 0.05). | |||||||
3.2.2 Differentiation of xylem and phloem cells
In the control trees, the first PC cells appeared on April 26 (DOY 116, Fig. 5C). During the next two weeks, the cells grew to their final full size, initiating the gradual SW phase on May 13 (DOY 133, Fig. 5E). The cell lignification started during the following two weeks. The first fully matured tracheids were observed on May 28 (DOY 147, Fig. 5G). The fully lignified tree ring was observed on October 5 (DOY 278).
Above the stem girdling, the cells continued to form while the differentiation lasted until the third week of September (DOY 264), likewise with the control trees, when all xylem cells had already formed a SW. The Kruskal–Wallis test revealed the SW formation lasted significantly longer above stem girdling than in control trees (Table 1, p = 0.029 resp. p = 0.032). In the following month, the tracheids were completely lignified (October 23, DOY 296). The tree-ring above the stem girdling contained fully matured latewood tracheids (Fig. 3A).
Below the stem girdling, the formation and growth of cells ceased one week after the treatment (Figs. 5C, E). The differentiation of the tissues was reduced to a point that many tracheids failed to form the secondary cell wall until the end of the growing season. Hence, fully mature latewood tracheids were absent below the stem girdling.
The EP formation (Fig. 6C, Table 2) started one week after the cambial reactivation (April 22, DOY 112) for all trees. Phloem cell formation, above the girdling, ceased three weeks earlier than in the control trees, although this difference was not significant (p = 0.089).
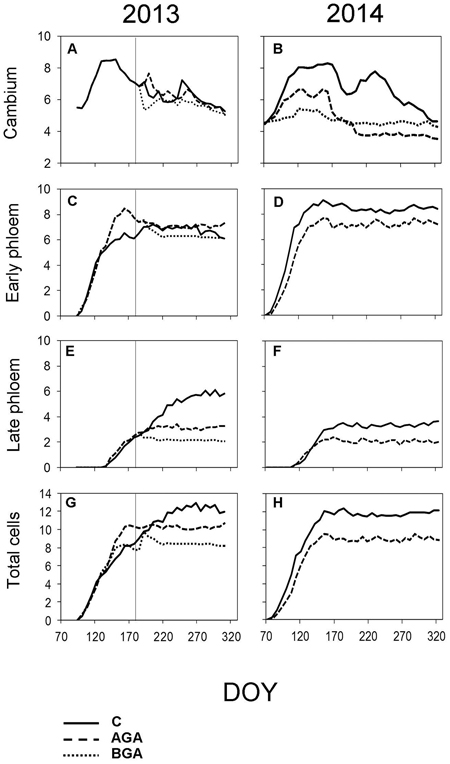
Fig. 6. Dynamics of phloem formation and cambial activity during the 2013 and 2014 growing seasons. Number of cells within cambial zone (A, B), number of EP cells (C, D) and LP cells (E, F) and the total cell numbers within fully formed annual phloem increment (G, H). C – control trees (solid line); AGA – above girdling area (dashed line); BGA – below girdling area (dotted line). DOY – day of the year (70: March 11, 120: May 30, 170: June 19, 220: August 8, 270: September 27, 320: November 16). Solid vertical line – date of girdling.
| Table 2. Timings of phloem cell differentiation phases in six control and six girdled (above girdling) trees (2013 and 2014). | |||||||
| 2013 | 2014 | ||||||
| Parameters (DOY) | Control | AGA | K–W test | Control | AGA | K–W test | |
| EP | 112 ± 4 | 114 ± 3 | 0.847 | 88 ± 4 | 95 ± 4 | 0.014 | |
| LP | 144 ± 5 | 148 ± 7 | 0.473 | 129 ± 8 | 122 ± 6 | 0.107 | |
| CLP | 232 ± 16 | 214 ± 17 | 0.089 | 181 ± 14 | 174 ± 4 | 0.108 | |
| EP duration (days) | 41 ± 5 | 43 ± 11 | 0.737 | 47 ± 10 | 35 ± 9 | 0.038 | |
| LP duration (days) | 88 ± 17 | 67 ± 16 | 0.075 | 51 ± 14 | 52 ± 8 | 0.924 | |
| Total duration (days) | 128 ± 16 | 110 ± 17 | 0.061 | 98 ± 13 | 87 ± 4 | 0.059 | |
| P-values for Kruskal–Wallis test (H0 – Medians of all groups are equal, if p < alpha (0.05) > rejecting H0). DOY – day of year; Control – control trees; AGA – above girdling area; K–W test – results (p-values) of Kruskal–Wallis test; EP – onset of early phloem formation; LP – onset of late phloem formation and cessation of EP; CLP – cessation of LP. Bold – statistically significant difference (alpha = 0.05). | |||||||
3.2.3 Radial increment (Gompertz function)
In the control trees, the total radial increment (TOTAL) contained 43.7 xylem cells according to the Gompertz function. The daily rate of xylem cell production was 0.21 cells per day. The maximum daily rate of xylem cell production (0.30 cells per day) was observed on June 26 (DOY 177). The control trees needed 212 days to form the TOTAL number of xylem cells (Fig. 7, Table 3).
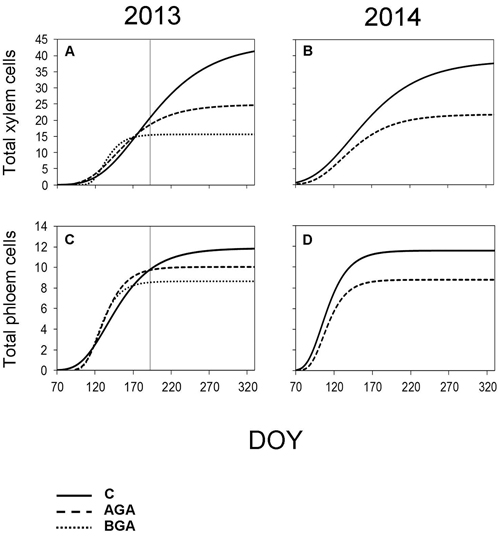
Fig. 7. Dynamics of xylem and phloem formation fitted by the Gompertz function during the 2013 and 2014 growing seasons. S-shaped curve – Gompertz function. C – control trees (solid line); AGA – above girdling area (dashed line); BGA – below girdling area (dotted line). DOY – day of the year (70: March 11, 120: May 30, 170: June 19, 220: August 8, 270: September 27, 320: November 16). Solid vertical line – date of girdling.
| Table 3. Dynamics of xylem formation from the parameters of the Gompertz function during 2013 and 2014 in six control and six girdled trees (above, below). | |||||||
| 2013 | 2014 | ||||||
| Control | Girdled | Control | Girdled | ||||
| Parameters | AGA | BGA | AGA | BGA | |||
| Final cell number | 43.71 | 24.84 | 15.63 | 38.60 | 21.80 | - | |
| Daily cell rate of xylem formation | 0.21 | 0.17 | - | 0.19 | 0.15 | - | |
| Maximum daily cell rate | 0.30 | 0.24 | - | 0.28 | 0.22 | - | |
| Day of maximum daily cell rate (DOY) | 176.57 | 145.65 | - | 142.34 | 130.76 | - | |
| Duration of xylem formation (days) | 212.17 | 149.41 | - | 202.74 | 145.04 | - | |
| AGA – above girdling area; BGA – below girdling area. | |||||||
Above the stem girdling, half the number of cells, compared with the control trees, were formed (24.8 cells). The maximum daily rate of xylem cell production (0.24 cells per day) was recorded one month earlier (May 26, DOY 146) than in the control trees, whereas the rate of the xylem cell production was 0.17 cells per day. We found a noticeably reduced number of days (149) needed to form the TOTAL of xylem cells above the girdling. Meanwhile, only 15.6 xylem cells were formed below the girdling.
The maximum daily rate of phloem cell production, above the girdling, occurred approximately one week earlier than in the control trees (May 6, DOY 126). Although, the final number of phloem cells was similar to the control trees (10.1 cells), nevertheless, the daily rate of the phloem cell production was higher (0.13 cells per day), probably due to the shorter duration of phloem formation: 77 days. Finally, we observed 8.7 phloem cells below the girdling (Fig 7, Table 4).
| Table 4. Dynamics of phloem formation from the parameters of the Gompertz function during 2013 and 2014 in six control and six girdled trees (above, below). | |||||||
| 2013 | 2014 | ||||||
| Control | Girdled | Control | Girdled | ||||
| Parameters | AGA | BGA | AGA | BGA | |||
| Final cell number | 11.87 | 10.05 | 8.65 | 11.59 | 8.78 | - | |
| Daily cell rate of phloem formation | 0.09 | 0.13 | - | 0.15 | 0.13 | - | |
| Maximum daily cell rate | 0.13 | 0.19 | - | 0.22 | 0.19 | - | |
| Day of maximum daily cell rate (DOY) | 135.26 | 125.53 | - | 103.69 | 105.64 | - | |
| Duration of phloem formation (days) | 138.12 | 76.80 | - | 77.12 | 67.30 | - | |
| AGA – above girdling area; BGA – below girdling area. | |||||||
3.3 Growth period following treatment (2014)
3.3.1 Cambial activity
In the control trees, 5.4 ± 0.5 cambial cells were recorded during dormancy (Fig. 5B). The cambium was reactivated almost three weeks earlier in 2014 than in 2013 (from March 28 to April 3), while the sum of the effective temperature barely reached 116.6 °C (Fig. 4D). The number of cells in the cambial zone rapidly reached 8.1 ± 0.9 cells by May, maintained this number during the whole month and finally peaked (8.3 ± 1.8 cells) on June 12 (DOY 163). In July, the number started decreasing to 6.5 ± 0.4 cells, but the cambium was reactivated again at the beginning of August, increasing to 7.4 ± 0.9 cells. By mid-September, we recorded another decrease in cell numbers, which lead to the final cessation of the cambial activity (October 4, DOY 277) when it finally returned to the number of cells of the dormant period.
In the girdled trees, the cambial zone above the stem girdling was activated during the first week of April (DOY 93), one week later than in the control trees (Table 1, p = 0.026). During the spring, the number of cambial cells above the girdling was lower than in the control trees (6 cells) during April. The highest number (6.6 ± 0.8 cells) was recorded in the first week of May (DOY 121−128) and it was maintained until mid-June (June 12, DOY 163). Then the number of cambial cells decreased to 5.2 ± 0.3 cells for two weeks, before the final cessation of the cambial activity (June 17, DOY 168), which occurred significantly earlier than in the control trees (Table 1, p = 0.003). No cambial cells derivatives were formed below the stem girdling (Figs. 2H, 3D).
3.3.2 Differentiation of xylem and phloem cells
In the control trees, the cambial reactivation occurred on March 28 (DOY 87) (Figs. 5B, 6B). The PC phase began approximately seven days later and lasted for three weeks. The xylem cell differentiation lasted until September 25 (DOY 268) when all cells had completed the SW formation. Eventually, all cells were fully lignified during the third week of October (DOY 293).
Above the girdling, most of the xylem differentiation phase started significantly later and lasted for a shorter period than in the control trees, as demonstrated by Kruskal–Wallis test (Table 1). The xylem cells of PC began to form during the week April 3–10 (DOY 93–100), one week later than in the control trees. The onset of the SW phase occurred in the last week of April (April 29, DOY 119), coinciding with the control trees. The first fully matured tracheids (MT) appeared one week after the onset of SW (May 7, DOY 127). In June, the production of new cells slowed down and finally ceased on June 23 (DOY 174), which is significantly different to the control trees. The xylem cells which already had a formed secondary wall, were lignified within the following two weeks, significantly faster than the control trees (p = 0.004, Fig. 5F). The tree-ring above the stem girdling formed no latewood tracheids (Fig. 3H).
The control trees started forming the EP immediately after the cambium reactivation (DOY 88). The first row of EP above the stem girdling was observed in the week April 3 to 10 after the cambial reactivation, significantly later than in the control trees (Table 2, p = 0.014). The initiation of the LP phase occurred in the first week of May above the stem girdling and during the following week in the control trees. The cessation of phloem cell formation occurred on June 30 (DOY 181) in the control trees which, was one week earlier than in the area above the girdling.
The cambium below the girdling was not activated. Therefore, neither xylem nor phloem formation occurred (Figs. 2, 3, 5, 6).
3.3.3 Radial increment (Gompertz function)
According to the Gompertz function, the TOTAL radial increment of the control trees contained 38.6 xylem cells (Table 3). The maximum daily rate of xylem cell production (0.28 cells per day) was recorded on May 22 (DOY 142) while the average daily rate of xylem cell production was 0.19 cells per day. Furthermore, it took 203 days to form the TOTAL of the xylem cells.
The TOTAL found in the above the girdling area contained less xylem cells than in the control trees (21.8 cells). The maximum daily rate of xylem cell production occurred earlier (May 11, DOY 131). Although both the maximum and average daily rate of xylem cell production was found to be similar to the control trees in 2014, the xylem cell formation period was shortened to 145 days.
The control trees reached the maximum daily rate of production of phloem cells on April 14 (DOY 104). It took 77 days to form the TOTAL (11.6) phloem cells. The rate of phloem cell production in the control trees and in the above the girdling area was similar (0.15 and 0.13 phloem cells per day, respectively).
The TOTAL of phloem cells (Table 4) was found to be smaller (8.8 cells) above the girdling and the duration of xylem cell formation was shorter (67 days). The maximum daily rate of phloem cell production in the control trees and above the girdling was recorded around the same time in April (DOY 104 and 106 respectively) and with similar values (0.15 and 0.13 phloem cells per day, respectively).
However, in 2013, the derivatives produced by the Gompertz function corresponded poorly with reality, because the model was obviously not flexible enough to adapt and display the sudden changes in growth rates due to a stress factor being applied as the growing season was in progress.
4 Discussion
To our knowledge, this is the first study on xylem and phloem formation in Scots pine conducted in the Czech Republic. Furthermore, it is the first attempt to apply stem girdling to 80-year-old Scots pine trees to examine the impact of this artificial stress factor on the cambial reactivation timings, the plasticity of the xylem and phloem formation and the change in the final structure of the tree rings above and below the girdling.
In the control trees, the xylem and phloem formation was in line with other studies on Scots pine wood formation conducted at various sites in Europe (e.g. Antonova and Stasova 2006; Gruber et al. 2010; Swidrak et al. 2014). Nevertheless, we noticed a cambial latency in all the examined trees, (control and girdled trees i.e. above the girdling) occurred after a summer drought. In any case, several researchers have presented, in detail, the response of the species to extreme drought events (e.g. Gruber et al. 2010; Lebourgeois et al. 2011; Eilmann et al. 2011; Swidrak et al. 2011).
The selection of the girdling date was important for the monitoring of cell differentiation and the final ceasing of growth. Daudet et al. (2005) girdled the trees early in the growing season (DOY 193). Tombesi et al. (2014) claimed that the earlier the girdling was induced, the more obvious the decrease in midday stem water potential was as well as the impact on shoot growth. Choi et al. (2010) suggested that stem girdling performed in April or June allowed trees to reduce vegetative growth, increase fruit set and accelerate fruit coloration, concluding that, in particular, a June girdling (after full bloom) could control tree vitality. The stem girdling we performed, during the first week of July (DOY 192), had little effect on the earlywood formation, since most of the earlywood tracheids had already completed their secondary wall formation, almost lignified and already formed LP cells. Hence, there was a minor effect, from the girdling, on the hydraulic architecture of the stem during 2013, especially considering the proportion of sapwood of the Scots pine. However, there was a significant effect on latewood production.
In line with Lopez et al. (2015), we found that the cambial activity ceased below the girdling, terminating any further cell formation. Barbosa and Wagner (1989) stated that the severity of the effects depended greatly on when the girdling was performed during the growth period, on the localized hormones synthesis and transport, as well as the weather conditions or the genetic predisposition of the species. Recent studies reported that the induced stress caused by girdling stopped the growth of already formed cells, proving the depletion of the soluble non-structural carbohydrates from the living cells below the girdling (Dunn and Lorio 1992; Mei et al. 2015). Nevertheless, Wilson (1968) found enough carbohydrates left for cell differentiation and lignification in the already formed cells in white pine (Pinus strobus L.).
In our study, despite the immediate cessation of cambial activity after girdling, we noticed a short new cambial reactivation which resulted in the exiguous increment of one discontinuous latewood row. This cambial reactivation occurred during the second half of August after a drought event in both examined years (2013 and 2014). Apparently, root carbon may be mobilized for growth even several weeks after the girdling (Mei et al. 2015). However, the cell differentiation below the girdling, was unquestionably carried out with a significantly lower intensity, obviously due to the reduced supply of assimilates and plant hormones (auxin and giberilin), which strongly define the cell formation and the cell wall lignification process (Tokunaga et al. 2006; Sorce et al. 2013). We observed that the tracheids derived by cambium before or immediately after girdling, were fixed in the PC phase. Thus, below the girdling, typical thick-wall latewood tracheids were not formed. This coincided with Wilson (1968), who also reported the absence of any latewood increment below girdling. Nevertheless, above the girdling, the latewood tracheids continued developing and differentiating, while the cambial division was less intensive. Furthermore, the cessation timing of xylem and phloem formation was found to be different, as the xylem formation in the girdled trees finished 18 days later than in the control, whereas the phloem formation ceased three weeks earlier. In both cases, fewer cells were formed after the girdling, compared with the control trees, probably due to lower transport of water and assimilates. Our finding contradicts that of Wilson (1968), who reported an increase in the phloem cell formation rate (from 0.11 to 0.27 cells) after girdling.
We found that the Gompertz function failed to depict the growth rate after girdling, since the model assumes that trees would grow under similar conditions. In 2013, the Gompertz function falsely showed a considerably lower number of days needed for the formation of most the majority of the xylem cells above the girdling compared with the control (149 and 212 days respectively). Nevertheless, the model fitted better with 2014 growth rates.
Johnsen et al. (2007) mentioned that girdling is destructive and irreversible. Ultimately, our study showed that the Scots pine trees managed to survive for two years. This surviving period could be attributed to several important factors ensuring the transport of nutrients to roots, such as the basal shoot development or the depletion of nutrient reserves in the roots. Mycorrhizal associations or root grafts could also provide collateral support to girdled trees (Noel 1970; Barbosa and Wagner 1989). Nevertheless, this would rarely be long-lasting as the active xylem tissue of the tree is severely destroyed by girdling (Moore 2013).
5 Conclusions
The knowledge of tree survival mechanisms and the underlying processes leading to death due to stress factors could be a useful tool for physiologists, ecologists and foresters. The girdling stress inhibits the cambial activity and hence limits or even completely stops the wood growth increment (Stone 1974; Larson 1994; Wilson and Gartner 2002). When we subjected Scots pine trees to girdling stress for two subsequent growing seasons, the cambial activity was inhibited above, and suddenly stopped below, the stem girdling, immediately after applying the stress factor. Above the stem girdling, the cell formation and the tissue differentiation continued, until the end of the growing season, at a less vigorous rate compared with the control trees. During the second growing season, the cambial zone below the stem girdling was not reactivated. All the girdled trees died during 2014, one year after the girdling. In the future, a morphometric analysis of the cells formed after girdling, examining the processes of potential swelling or regenerating would be of great interest.
Acknowledgements
This experimental research was supported by the Internal Grand Agency of Faculty of Forestry and Wood Technology (IGA, 42/2014) and by the project „The Effect of stress on physiology, anatomy and xylem xylogenesis pine forest“ (LD COST CZ, No. LD13017).
In addition, we would like to thank the anonymous reviewers for their valuable comments and suggestions for improving the quality of the paper. We thank David Millar for language editing.
References
Abramoff M.D., Magalhães P.J., Ram S.J. (2004). Image processing with imagej. Biophotonics International 11(7): 36–42. ISSN 1081-8693.
Antonova G.F., Stasova V.V. (1993). Effects of environmental factors on wood formation in Scots pine stems. Trees 7(4): 214–219. https://doi.org/10.1007/BF00202076.
Antonova G.F., Stasova V.V. (2006). Seasonal development of phloem in scots pine stems. Physiology of Plant Development 37(5): 306–320. https://doi.org/10.1134/S1062360406050043.
Antonova G.F., Cherkashin V.P., Stasova V.V., Varaksina T.N. (1995). Daily dynamics in xylem cell radial growth of Scots pine (Pinus sylvestris L.). Trees 10(1): 24–30. https://doi.org/10.1007/BF00197776.
Augusti M., Andreu I., Juan M., Almela V., Zacarias L. (1998). Effects of ringing branches on fruit size and maturity of peach and nectarine cvs. Journal of Horticultural Science and Biotechnology 73(4): 530–540. https://doi.org/10.1080/14620316.1998.11511011.
Barbosa P., Wagner M.R. (1989). Introduction to forest and shade tree insects. https://doi.org/10.1016/B978-0-12-078146-1.50001-8.
Bogino S., Fernández Nieto M.J., Bravo F. (2009). Climate effect on radial growth of Pinus sylvestris at its southern and western distribution limits. Silva Fennica 43(4): 609–623. https://doi.org/10.14214/sf.183.
Bormann F.H. (1966). The structure, function, and ecological significance of root grafts in Pinus strobus L. Ecological Monographs 36(1): 1–26. https://doi.org/10.2307/1948486.
Choi S.T., Song W.D., Park D.S., Kang S.M. (2010). Effect of different girdling dates on tree growth, fruit characteristics and and reserve accumulation in a late-maturing persimmon. Scientia Horticulturae 126(2): 152–155. https://doi.org/10.1016/j.scienta.2010.06.026.
Chytrý M., Kučera T., Kočí M., Grulich V., Lustyk P. (2010). In: Katalog biotopů České republiky (eds.). Agentura ochrany přírody a krajiny ČR, Praha. p. 206–214.
Crane J.C., Cambell R.C. (1957). The comperative effectiveness of girdling and 2,4,5 Trichlorophenoxyacetic acid for increasing size and hastening maturity of apricots. Proceedings of the American Society for Horticultural Science 69: 165–9.
Cuny H.E., Rathgeber C.B.K., Lebourgeois F., Fortin M., Fournier M. (2012). Life strategies in intra-annual dynamics of wood formation: example of three conifer species in a temperate forest in north-east France. Tree Physiology 32(5): 612–625. https://doi.org/10.1093/treephys/tps039.
Cuny H.E., Rathgeber C.B.K., Frank D., Fonti P., Fournier M. (2014). Kinetics of tracheid development explain conifer tree-ring structure. New Phytologist 203(4): 1231–1241. https://doi.org/10.1111/nph.12871.
Cuny H.E., Rathgeber C.B.K. Frank D., Fonti P., Makinen H., Prislan P., Rossi S., del Castillo E.M., Campelo F., Vavrcik H., Camarero J.J., Bryukhanova M.V., Jyske T., Gricar J., Gryc V., Luis M.D., Vieira J., Cufar K., Kirdyanov A.V., Oberhuber W., Treml V., Huang J.G., Li X., Swidrak I., Deslauriers A., Liang E., Nojd P., Gruber A., Nabais C., Morin H., Krause C., King G., Fournier M. (2015) Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nature Plants 1(15160). https://doi.org/10.1038/nplants.2015.160.
Daudet F.A., Ameglio T., Cochard H., Archilla O., Lacointe A. (2005). Experimental analysis of the role of water and carbon in tree stem diameter variations. Journal of Horticultural Science and Biotechnology 56(409): 135–144. https://doi.org/10.1093/jxb/eri026.
Day K.R., DeJong T.M. (1999). Improving fruit size: thinning and girdling nectarines, peaches and plums. Compact Fruit Tree 32: 49–51.
De Schepper V., Steppe K. (2011). Tree girdling responses simulated by a water and carbon transport model. Annals of Botany-London 108(6): 1147–1154. https://doi.org/10.1093/aob/mcr068.
Deslauriers A., Rossi S., Anfodillo T., Saracino A. (2008). Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy.Tree Physiology 28(6): 863–871. https://doi.org/10.1093/treephys/28.6.863.
Domec J.C., Pruyn M.L. (2008). Bole girdling affects metabolic properties and root, trunk and branch hydraulics of young ponderosa pine trees. Tree Physiology 28(10): 1493–1504. https://doi.org/10.1093/treephys/28.10.1493.
Dunn J.P., Lorio P.L. (1992). Effects of bark girdling on carbohydrate supply and resistance of loblolly pine to southern pine beetle (Dendroctonus frontalis Zimm.) attack. Forest Ecology and Management 50(3–4): 317–330. https://doi.org/10.1016/0378-1127(92)90345-A.
Evert R.F. (2006). Esau‘s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development. John Wiley & Sons, New Jersey. 601 p. https://doi.org/10.1002/0470047380.
Eilmann B., Zweifel R., Buchmann N., Pannatier E.G., Rigling A. (2011). Drought alters timing, quantity, and quality of wood formation in scots pine. Journal of Experimental Botany 62(8): 2763–2771. https://doi.org/10.1093/jxb/erq443.
Fernandez-Escobar R., Martin R., Lopez-Rivares P., Paz Suarez M. (1987). Girdling as a means of increasing fruit size and earliness in peach and nectarine cultivars. Journal of Horticultural Science and Biotechnology 62(4): 463–468. https://doi.org/10.1080/14620316.1987.11515807.
Fishman S., Genard M., Huguet J.G. (2001). Theoretical analysis of systematic errors introduced by a pedicel-girdling technique used to estimate separately the xylem and phloem flows. Journal of Theoretical Biology 213(3): 435–446. https://doi.org/10.1006/jtbi.2001.2442.
Gričar J. (2007). Xylo- and phloemogenesis in Silver fir (Abies alba Mill.) and Norway spruce (Picea abies (L.) Karst.). Slovenian Forestry Institute, Ljubljana. 106 p.
Gruber A., Strobl S., Veit B., Oberhuber W. (2010). Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiology 30(4): 490–501. https://doi.org/10.1093/treephys/tpq003.
Harris I., Jones P.D., Osborn T.J., Lister D.H. (2013). Updated high-resolution grids of monthly climatic observations – the CRU TS3.10 dataset. International Journal of Climatology 34(3): 623–642. https://doi.org/10.1002/joc.3711.
Johnsen K., Maier C., Sanchez F., Anderson P., Butnor J., Waring R. (2007). Physiological girdling of pine trees via phloem chilling: proof of concept. Plant, Cell & Environment 30(1): 128–134. https://doi.org/10.1111/j.1365-3040.2006.01610.x.
Jordan M.O., Habib R. (1996). Mobilizable carbon reserves in young peach trees as evidenced by trunk girdling experiments. Journal of Experimental Botany 47(1): 79–87. https://doi.org/10.1093/jxb/47.1.79.
Jyske T., Mäkinen H., Kalliokoski T., Nöjd P. (2014). Intra-annual tracheid production of Norway spruce and scots pine across a latitudinal gradient in Finland. Agricultural and Forest Meteorology 194: 241–254. https://doi.org/10.1016/j.agrformet.2014.04.015.
Kelly D.L., Connolly A.A. (2000). Review of the plant communities associated with Scots pine (Pinus sylvestris L.) in Europe, and an evaluation of putative indicator/specialist species. Forest Systems 9: 15–39. http://revistas.inia.es/index.php/fs/article/view/674/671. [Cited 30 Nov. 2016].
Larson P.R. (1994). The vascular cambium. Development and structure. Springer-Verlag, Berlin, Heidelberg, New York. p. 521–525.
Lebourgeois F., Mérian P. (2011). Has sensitivity of forest species to climate changed in the 20th century? [La sensibilité au climat des arbres forestiers a-t-elle changé au cours du XXième siècle?] Revue Forestière Française 63(1): 17–32. ISSN 00352829. [In French].
Lewis L.N., McCarty C.D. (1973). Pruning and girdling of citrus. In: The citrus industry Vol. III (Reuther W.) (ed.). University of California, Berkeley, USA.
Lilleland O., Brown J.G. (1936). Growth study of the apricot fruit. III. The effect of girdling. Proceedings of the American Society for Horticultural Science 34: 264–71.
López R., Brossa R., Gil L., Pita P. (2015). Stem girdling evidences a trade-off between cambial activity and sprouting and dramatically reduces plant transpiration due to feedback inhibition of photosynthesis and hormone signaling. Frontiers in Plant Science 6(285): 1–13. https://doi.org/10.3389/fpls.2015.00285.
MacDougal D.T. (1943). The effect of girdling on pines. American Journal of Botany 30(9): 715–719. https://doi.org/10.2307/2437719.
Martinez del Castillo E., Longares L.A., Gričar J., Prislan P., Gil-Pelegrín E., Čufar K., Luis M. (2016). Living on the edge: contrasted wood-formation dynamics in Fagus sylvatica and Pinus sylvestris under Mediterranean conditions. Frontiers in Plant Science 7(370): 1–10. https://doi.org/10.3389/fpls.2016.00370.
Maunoury-Danger F., Fresneau C., Eglin T., Berveiller D., François C., Lelarge-Trouverie C., Damesin C. (2010). Impact of carbohydrate supply on stem growth, wood and respired CO2 δ13C: assessment by experimental girdling. Tree Physiology 30(7): 818–830. https://doi.org/10.1093/treephys/tpq039.
Mei L., Xiong Y., Gu J. (2015). Whole-tree dynamics of non-structural carbohydrate and nitrogen pools across different seasons and in response to girdling in two temperate trees. Oecologia 177(2): 333–344. https://doi.org/10.1007/s00442-014-3186-1.
Ministry Report (2014). Report on forest and forestry in the Czech Republic by 2013. Ministry of Agriculture of the Czech Republic, Prague, Czech Republic. 30 p. http://eagri.cz/public/web/file/353116/Zprava_o_stavu_lesa_2013_ENG.pdf.
Morandi B., Rieger M., Grappadelli L.C. (2007). Vascular flows and transpiration affect peach (Prunus persica Batsch.) fruit daily growth. Journal of Experimental Botany 58(14): 3941–3947. https://doi.org/10.1093/jxb/erm248.
Moore G.M. (2013). Ring-barking and girdling: how much vascular connection do you need between roots and crown? Burnley College, University of Melbourne, Richmond. 3121 p.
Münch E. (1938). Untersuchungen uiber die Harmonie der Baumgestalt. Jahrbücher für Wissenschaftliche Botanik 86: 581–673.
NCAR-UCAR (2016). The climate data guide. Boulder, USA. https://climatedataguide.ucar.edu.
Noel A.R.A. (1970). The girdled tree. Botanical Review 36: 162–195. https://doi.org/10.1007/BF02858959.
Novák J., Sádlo J., Svobodová-Svitavská H. (2012). Unusual vegetation stability in a lowland pine forest area (Doksy region, Czech Republic). The Holocene 22(8): 947–955. https://doi.org/10.1177/0959683611434219.
Oberhuber W., Swidrak I., Pirkebner D., Gruber A. (2011). Temporal dynamics of nonstructural carbohydrates and xylem growth in Pinus sylvestris exposed to drought. Canadian Journal of Forest Research 41(8): 1590–1597. https://doi.org/10.1139/x11-084.
Pang Y., Zhang J., Cao J., Yin S.Y., He X.Q., Cui K.M. (2008). Phloem transdifferentiation from immature xylem cells during bark regeneration after girdling in Eucommia ulmoides Oliv. Journal of Experimental Botany 59(6): 1341–1351. https://doi.org/10.1093/jxb/ern041.
Panshin A.J., Zeeuw C.D. (1980). Textbook of wood technology: structure, identification, properties, and uses of the commercial woods. McGraw-Hill Series in Forest Resources. 4th ed. Mcgraw-Hill, New York. 13 p.
Powell A.A., Cash H.J. (1985). Increase size with girdling. Fruit Grower 1: 12–14.
Plomion C., LeProvost G., Stokes A. (2001). Wood formation in trees. Plant Physiology 127: 1513–1523. https://dx.doi/10.1104/pp.010816.
Prislan P., Gričar J., de Luis M., Novak K., Martinez del Castillo E., Schmitt U., Koch G., Štrus J., Mrak P., Žnidarič M.T., Čufar K. (2016). Annual cambial rhythm in Pinus halepensis and Pinus sylvestris as indicator for climate adaptation. Frontiers in Plant Science 7(1923): 1–15. https://doi.org/10.3389/fpls.2016.01923.
Rathgeber C.B.K., Rossi S., Bontemps J.-D. (2011). Cambial activity related to tree size in a mature silver-fir plantation. Annals of Botany 108(3): 429–438. https://doi.org/10.1093/aob/mcr168.
Richardson D.M., Higgins S.I. (1998). Pines as invaders in the southern hemisphere. In: Richardson D.M. (ed.). Ecology and biogeography of Pinus. Cambridge University Press, Cambridge. p. 450–473.
Rivas F., Fornes F., Agustí M. (2008). Girdling induces oxidative damage and triggers enzymatic and non-enzymatic antioxidative defences in Citrus leaves. Environmental and Experimental Botany 64(3): 256–263. https://doi.org/10.1016/j.envexpbot.2008.07.006.
Romagnoli M., Cherubini M., Prislan P., Gričar J., Spina S., Čufar K. (2011). Main phases of wood formation in chestnut (Castanea sativa) in central Italy – comparison of seasons 2008 and 2009. Drvna Industrija 62(4): 269–275. https://dx.doi/10.5552/drind.2011.1124.
Rossi S., Deslauriers A., Morin H. (2003). Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia 21(1): 33–39. https://doi.org/10.1078/1125-7865-00034.
Rossi S., Anfodillo T., Menardi R. (2006). Trephor: a new tool for sampling microcores from tree stems. Iawa Journal 27(1): 89–97. https://doi.org/10.1163/22941932-90000139.
Rossi S., Morin H., Deslauriers A. (2012). Causes and correlations in cambium phenol-ogy: towards an integrated framework of xylogenesis. Journal of Experimental Botany 63(5): 2117–2126. https://doi.org/10.1093/jxb/err423.
Rossi S., Anfodillo T., Cufar K., Cuny H.E., Deslauriers A., Fonti P., Frank D., Gricar J., Gruber A., King G.M., Krause C., Morin H., Oberhuber W., Prislan P., Rathgeber C.B.K. (2013). A meta-analysis of cambium phenology and growth: linear and non-linear patterns in conifers of the northern hemisphere. Annals of Botany 112(9): 1911–1920. https://doi.org/10.1093/aob/mct243.
Ryan K.C. (1990). Predicting prescribed fire effects on trees in the Interior West. In: Alexander M.E., G.F. Bisgrove, technical coordinators. The Art and Science of Fire Management. Proceedings of the first Interior West fire council annual meeting and workshop, Kananaskis Village, Alberta, October 24-27, 1988. Forestry Canada Information Report NOR-X-309. p. 148–162.
Salleo S., Lo Gullo M.A., De Paoli D., Zippo M. (1996). Xylem recovery from cavitation – induced embolism in young plants of Laurus nobilis: a possible mechanism. New Phytologist 132(1): 47–56. https://doi.org/10.1111/j.1469-8137.1996.tb04507.x.
Salleo S., Trifilo P., Lo Gullo M.A. (2006). Phloem as possible major determinant of rapid cavitation reversal in stems of Laurus nobilis (laurel). Functional Plant Biology 33(11): 1063–1074. https://doi.org/10.1071/FP06149.
Schmitt C.L., Filip G.M. (2005). Understanding and defining mortality in western conifers. R6-FHP-1-05, USDA Forest Service, Pacific Northwest Region, Portland, OR. 17 p.
Schmitt U., Jalkanen R., Eckstein D. (2004). Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fennica 38(2): 167–178. https://doi.org/10.14214/sf.426.
Sellin A., Nigla A., Õunapuu E., Karusion A. (2013). Impact of phloem girdling on leaf gas exchange and hydraulic conductance in hybrid aspen. Biologia Plantarum 57(3): 531–539. https://doi.org/10.1007/s10535-013-0316-2.
Seo J.W., Eckstein D., Jalkanen R., Schmitt U. (2011). Climatic control of intra- and inter-annual wood-formation dynamics of scots pine in northern finland. Environmental and Experimental Botany 72(3): 422–431. https://doi.org/10.1016/j.envexpbot.2011.01.003.
Sorce C., Giovannelli A., Sebastiani L., Anfodillo T. (2013). Hormonal signals involved in the regulation of cambial activity, xylogenesis and vessel patterning in trees. Plant Cell Reports 32(6): 885–898. https://doi.org/10.1007/s00299-013-1431-4.
Stone E.L. (1974). The communal root system of red pine: growth of girdled trees. Forest Science 20: 294–305.
Swidrak I., Gruber A., Kofler W., Oberhuber W. (2011). Effects of environmental conditions on onset of xylem growth in Pinus sylvestris under drought. Tree Physiology 31(5): 483–493. https://doi.org/10.1093/treephys/tpr034.
Swidrak I., Gruber A., Oberhuber W. (2014). Xylem and phloem phenology in co-occurring conifers exposed to drought. Trees 28(4): 1161–1171. https://doi.org/10.1007/s00468-014-1026-x.
Taylor A.M. (1999). The effect of stem girdling on wood quality. M.Sc. thesis, University of New Brunswick, Fredericton, Canada. 153 p.
Tokunaga N., Uchimura N., Sato Y. (2006). Involvement of gibberellin in tracheary element differentiation and lignification in Zinnia elegans xylogenic culture. Protoplasma 228(4): 179–187. https://doi.org/10.1007/s00709-006-0180-4.
Tombesi S., Day K.R., Johnson R.S., Phene R., DeJong T.M. (2014). Vigour reduction in girdled peach trees is related to lower midday stem water potentials. Functional Plant Biology 41(12): 1336–1341. https://doi.org/10.1071/FP14089.
Vacek S., Vacek Z., Bílek L., Simon J., Remeš J., Hůnová I., Král J., Putalová T., Mikeska M. (2016). Structure, regeneration and growth of Scots pine (Pinus sylvestris L.) stands with respect to changing climate and environmental pollution. Silva Fennica 50(4) article 1564. https://doi.org/10.14214/sf.1564.
Wagener W.W. (1961). Guidelines for estimating the survival of fire-damaged trees in California. Miscellaneous paper 60. Pacific Southwest Forest and Range Experiment Station, U.S. Department of Agriculture, Forest Service.
Weinburger J.H., Cullinan F.P. (1932). Further studies on the relation between leaf area and size of fruit, chemical composition, and fruit bud formation in Elberta peaches. Proceedings of the American Society for Horticultural Science 29: 23–27.
Wilson B.F. (1968). Effect of girdling on cambial activity in white pine. Harvard University, Cabot Foundation. Petersham, Massachusetts. Canadian Journal of Botany 46(2): 141–146. https://doi.org/10.1139/b68-024.
Wilson B.F. (1998). Branches versus stems in woody plants: control of branch diameter growth and angle. Canadian Journal of Botany 76(11): 1852–1856. https://doi.org/10.1139/b98-156.
Wilson B.F., Gartner B.L. (2002). Effects of phloem girdling in conifers on apical control of branches, growth allocation and air in wood. Tree Physiology 22: 347–353. https://doi.org/10.1093/treephys/22.5.347.
Winkler A.J., Cook J.A., Kliewer W.M., Lider L.A. (1974). General viticulture. University of California Press, Berkeley, USA.
Wodzicki T.J. (1971). Mechanisms of xylem differentiation in Pinus silvestris L. Journal of Experimental Botany 22(3): 670–687. https://doi.org/10.1093/jxb/22.3.670.
Zwieniecki M.A., Holbrook N.M. (1998). Diurnal variation in xylem hydraulic conductivity in white ash (Fraxinus americana L.), red maple (Acer rubrum L.) and red spruce (Picea rubens Sarg.). Plant, Cell & Environment 21(11): 1173–1180. https://doi.org/10.1046/j.1365-3040.1998.00342.x.
Zwieniecki M.A., Hutyra H., Thompso M.V., Holbrook N.M. (2000). Dynamic changes in petiole specific conductivity in red maple (Acer rubrum L.), tulip tree (Liriodendron tulipifera L.) and northern fox grape (Vitis labrusca L.). Plant, Cell & Environment 23(4): 407–414. https://doi.org/10.1046/j.1365-3040.2000.00554.x.
Zwieniecki M.A., Melcher P.J., Field T.S., Holbrook N.M. (2004). A potential role for xylem-phloem interactions in the hydraulic architecture of trees: effects of phloem girdling on xylem hydraulic conductance. Tree Physiology 24(8): 911–917. https://doi.org/10.1093/treephys/24.8.911.
Wagener W.W. (1961). Guidelines for estimating the survival of fire-damaged trees in California. Miscellaneous paper 60. Pacific Southwest Forest and Range Experiment Station, U.S. Department of Agriculture, Forest Service.
Total of 87 references.

