The effect of climatic factors on height increment of Scots pine in sites differing by continentality in Latvia
Jansons Ā., Matisons R., Zadiņa M., Sisenis L., Jansons J. (2015). The effect of climatic factors on height increment of Scots pine in sites differing by continentality in Latvia. Silva Fennica vol. 49 no. 3 article id 1262. https://doi.org/10.14214/sf.1262
Highlights
- Height increment-climate relationships of Scots pine were assessed using dendrochronological techniques
- Annual height increment was significantly affected by climatic factors
- In western Latvia, temperature in preceding summer mainly affected height increment
- In eastern Latvia height increment was affected by previous autumn temperature
- During the 20th century, the effect of climatic factors has altered likely dues to climate change.
Abstract
Height growth of trees is a crucial parameter that influences the composition and productivity of forest stands and quality of timber; however, the relationships between annual height increment (HI) and climatic factors have been poorly studied. In this study, the effect of monthly mean temperature and precipitation sums on the HI of Scots pine in two sites in Latvia have been determined using dendrochronological techniques. Correlation and response function analyses were conducted for entire chronologies of HI and for 50-year intervals within them. Climatic factors significantly affected the HI of Scots pine; however, not only did the suite of significant factors differ between the sites, but the influence of these factors changed during the 20th century. In the site in western Latvia where climate is milder, temperature in the preceding summer was the main climatic determinant of HI. The effect of temperature in the dormant period and spring was significant during the first part of the 20th century, while the effect of temperature in the previous September and November has become significant since the second half of the 20th century. In the site in eastern Latvia where summers are hotter, HI has been affected by both temperature and water deficit related factors in the summer. However, since the later part of the 20th century, the effect of temperature in the previous October has intensified and become the main climatic determinant of HI.
Keywords
Pinus sylvestris;
dendroecology;
height growth reconstruction;
meteorological conditions;
Baltic region;
increment variation
- Jansons, LSFRI “Silava”, Rīgas str. 111, Salaspils, Latvia, LV2169 E-mail aris.jansons@silava.lv
-
Matisons,
LSFRI “Silava”, Rīgas str. 111, Salaspils, Latvia, LV2169
E-mail
robism@inbox.lv

- Zadiņa, LSFRI “Silava”, Rīgas str. 111, Salaspils, Latvia, LV2169 E-mail mara.zadina@silava.lv
- Sisenis, LUA Forestry Faculty, Akadēmijas str. 11, Jelgava, Latvia E-mail linards.sisenis@llu.lv
- Jansons, Forest Competence Centre, Dzērbenes str. 27, Riga, Latvia, LV1006 E-mail janis.jansons@silava.lv
Received 8 October 2014 Accepted 23 March 2015 Published 8 May 2015
Views 169241
Available at https://doi.org/10.14214/sf.1262 | Download PDF

1 Introduction
Climate is one of the main factors, which affects the growth of trees (Fritts 2001), distribution of tree species (Hickler et al. 2012) and development of forest ecosystems (Iverson and Prasad 1998; Maiorano et al. 2013). Accordingly, knowledge on temporal variations of climate-growth interactions is essential for understanding of possible changes in the productivity of stands and quality of timber (Savill et al. 1997; Stott and Loehle 1998; Kellomäki et al. 1999), and for an assessment of ecological and economic consequences of the climatic shifts currently underway (Hanewinkel et al. 2012).
The effect of climatic factors on tree growth is commonly assessed by the analysis secondary growth via diverse tree-ring proxies (McCarroll et al. 2003; Speer 2010), while the variation of primary (height) growth in relation to weather conditions has been studied scarcely (Pensa et al. 2005; Salminen and Jalkanen 2005, 2007; Dobbertin et al. 2010), likely due to the laborious data collection required. In addition, climatic factors affecting annual height increment (HI) and accordingly, tree-ring proxies, are expected to vary due to differences in timing of formation of the increments (Little and Pharis 1995; McCarroll et al. 2003) and patterns of carbon allocation (von Felten et al. 2007).
Height increment of trees, which influences productivity of stands and knottiness of wood (Kellomäki et al. 1999), is determined by the intensity and duration of height growth (Lanner 1976; Pallardy 2008) that are affected by genetic and environmental factors (Junttila 1986; Kaya et al. 1999; Pensa et al. 2005). The maximum tree height is determined by hydraulic properties of wood (Ryan and Yoder 1997) and as a tree reaches this limit, height growth gradually decreases resulting in an explicit age trend of HI (Volosyanchuk 2002; Salminen and Jalkanen 2005). Height growth of Scots pine occurs during the first part of the vegetation period and this process is considered to depend mainly on current assimilation rather than on nutrient reserves (Ericsson 1978; Hansen and Beck 1994; Lippu 1998; von Felten et al. 2007). Lanner (1976) proposed that HI of Scots pine is determined by two components: environmental (climatic) conditions during shoot elongation and the number of growth initials formed in the previous vegetation season. Height growth has been also related to the competitive ability and distribution of tree species (Stott and Loehle 1998; Reich and Oleksyn 2008).
The few studies conducted in Finland showed that under boreal conditions, HI of Scots pine has been limited by low temperature in the summer of the preceding year (Pensa et al. 2005; Salminen and Jalkanen 2005, 2007). However, in southern regions of its distribution, HI has been limited by drought-related factors (Mutke et al. 2003; Thabeet et al. 2009; Dobbertin et al. 2010). Our previous study showed that height growth of 40-year-old Scots pine in experimental plantations in Latvia has been affected by temperature in the current spring and precipitation in the previous summer (Jansons et al. 2013). Nevertheless, there is still insufficient knowledge about the climate-HI relationships of Scots pine and their temporal changes in the hemiboreal forest zone. Therefore, the aim of this study was to produce longer chronologies of HI for Scots pine in Latvia and to assess the climatic signals recorded in them. We hypothesized that the suite of climatic factors significant for HI differ between sites in the western and eastern region of Latvia, and that a shift of these factors has occurred during the 20th century.
2 Material and methods
2.1 Study area and sampling
Study material was collected in two naturally regenerated stands of Scots pine in the western (near Šķēde) and eastern (near Kalsnava) regions of Latvia (Fig. 1), which are located in the middle of the north-south range of Scots pine (EUFORGEN 2009). The sites were chosen because significant differences in radial growth patterns of trees have been found there (Jansons and Baumanis 2005; Matisons and Brūmelis 2012). Since only negligible genetic differences have been found for naturally regenerated Scots pine amongst regions of Latvia (Neimane et al. 2009), the diversity of these growth patterns is most likely environmentally determined.
Fig. 1. Location of the studied sites (grey squares).
The studied stands were growing on nutrient-poor, acidic (pH ~ 3), sandy soils in the Myrtillosa forest type (according to the national classification established by Bušs 1976). The age of the stands (according to the inventory) was approximately 110 and 100 years in the western and eastern regions, respectively. The relief was flat and the elevation was about 100 m a.s.l. In both regions, climatic conditions are mild, determined by the dominant western winds bringing cool and moist air masses from the Atlantic and the Baltic Sea, with continentality increasing eastwards. Mean annual temperature ranges from +6.1 to +5.4 °C in the western and eastern regions, respectively. January is the coldest and July is the warmest month, with mean monthly temperature ranging from –3.3 to –6.2 °C and from +16.6 to +17.3 °C in the western and eastern regions, respectively. The vegetation period (when mean diurnal temperature is above +5 °C) extends for about 185 and 175 days in the western and eastern regions, respectively. Mean annual precipitation in the western and eastern regions is about 660 and 620 mm, respectively. Approximately 35–40% of the annual precipitation falls during the summer period (June–August), resulting in a positive water balance (Krams and Ziverts 1993). Global climatic changes are particularly reflected as an increase of autumn-spring temperature (Lizuma et al. 2007), which has raised by 1.03 and 1.33 °C within these regions, during the 20th century. Although annual precipitation has no explicit trend (Briede and Lizuma 2007), the distribution of precipitation is becoming more heterogeneous and longer precipitation-free periods have been observed in summer (Avotniece et al. 2010).
In each site, 20 dominant pines with undamaged stems (top has not been lost) were selected and felled. The cutting was performed as low as possible. The felled trees were limbed and their stems were cut longitudinally (at ~ 3 cm distance from the pith). All whorls along the stem were identified on the cut surface of logs and additional cutting was applied to expose the origins of whorls (on the pith). The height of whorls (above the stem base) was measured with a precision of one centimetre. Three stem disks containing whorls from various heights (~ 1.3, 10 and 18 m) were collected from each log and used for dating of the height increments. Sampling was done during the dormant period in November of 2013 and January of 2014 in the eastern and western region of Latvia, respectively.
2.2 Data analysis
A time series of annual height increment (HI) for each tree was calculated based on the height of whorls. As the most recent whorls (treetops) were often lost during the felling, the time series were dated according to the age of the collected stem discs. All series were crossdated and their quality was checked first by graphical inspection, then statistically using the program COFECHA (Grissino-Mayer 2001). Time series, which showed low agreement (mean correlation and Gleichläufigkeit (GLK) with the rest of series below 0.35 and 0.50, respectively) were rejected from further analysis rather than corrected. Mean interseries correlation, autocorrelation, sensitivity, GLK and expressed population signal (EPS) indices (Wigley et al. 1984) were calculated in the program R (R Core Team 2013) using library “dplR” (Bunn 2008). The EPS indices were calculated for the detrended (using spline with wavelengths of 64 years and 50% frequency cut-off) time series of HI.
Considering that time series of HI contain high autocorrelation (Salminen and Jalkanen 2005; Jansons et al. 2013), residual chronologies for each site were produced using the program ARSTAN (Cook and Holmes 1996). Double detrending with negative exponential curve and cubic spline with rigidity of 128-years and 50% frequency cut-off level and autoregressive modelling was applied. The similarity of produced chronologies was described by GLK and Pearson correlation coefficients.
Climate-HI relationships were assessed using bootstrapped Pearson correlation and response function analyses between the residual chronologies of HI and climatic data, in program DendroClim2002 (Biondi and Waikul 2004). The analysis was conducted for the whole periods covered by HI chronologies and for 50-year moving intervals, to assess temporal changes in climate-growth relationships. Climate data were obtained from the Climatic Research Unit of University of East Anglia. High-resolution gridded datasets were used (Harris et al. 2014). Mean monthly temperature and precipitation sums were obtained for points located at the distance < 10 km from the studied stands. Considering that the effect of weather conditions on height growth might have a certain lag (Salminen and Jalkanen 2005, 2007), climatic data were divided into time windows from the September of two years prior to formation of height increment (t-2) to the August of the year of formation of the increment (t).
3 Results
3.1 Datasets
After the crossdating and quality check, 75% and 65% of the measured time series of HI from the western and eastern sites, respectively, showed good agreement and were used for further analysis (Table 1). The period covered by the crossdated time series was longer in the western site since the stand was older (Fig. 2). Sample depth of the eastern dataset was high during most of the period, while the replication of the western dataset has been gradually decreasing since 1969. An age trend was present in the time series of both sites (Fig. 2 A, B) and annual variation of mean HI was rather low. Common signatures (increases or decreases in most of the series in a certain year) amongst the crossdated time series were more expressed in the eastern site, while the individuality of variation of HI amongst trees was stronger in the western site, particularly since the 1970s. Common signatures as decreased HI in 1930, 1957 and 1979, were observed in both sites.
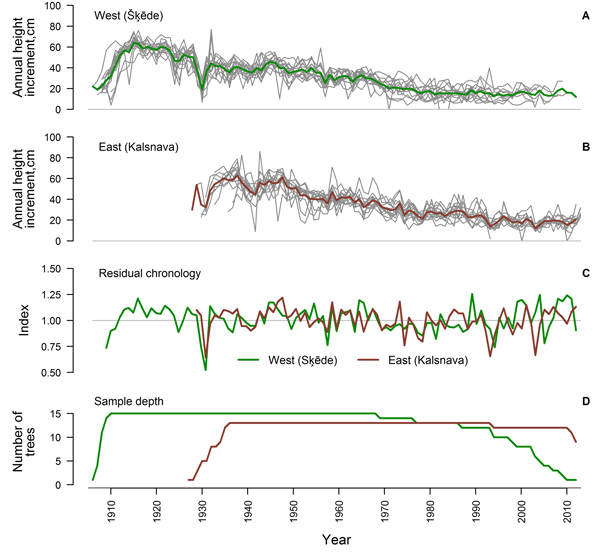
Fig. 2. Crossdated time series of annual height increment of Scots pine for the western (Šķēde) (A) and eastern (Kalsnava) (B) site (mean values shown by thick line), residual chronologies of annual height increment (C) and sample depth of the datasets (D).
Statistics of the crossdated datasets were similar for both sites (Table 1) with a high (> 0.80) mean autocorrelation of datasets and a mean sensitivity of approximately 0.20. Mean interseries correlation was higher in the western site. The EPS values calculated for the entire period exceeded 0.85 for both datasets and residual chronologies were successfully produced for each site (Fig. 2 C). The GLK of chronologies was 0.65 and correlation between them was rather weak (r = 0.37), suggesting different sources of variation, particularly during the recent four decades (Fig. 2 C).
| Table 1. Statistics of crossdated datasets of annual height increment (HI) of Scots pine for the western (Šķēde) and eastern (Kalsnava) site. Abbreviations: GLK – Gleichläufigkeit and EPS – expressed population signal (Wigley et al. 1984). | ||
| Western (Šķēde) | Eastern (Kalsnava) | |
| Number of crossdated time series | 15 | 13 |
| Covered period | 1906–2012 | 1927–2013 |
| Minimum HI, cm | 2 | 2 |
| Maximum HI, cm | 77 | 86 |
| Mean HI, cm | 33.21 | 34.28 |
| Standard deviation of HI, cm | 15.70 | 15.40 |
| Mean interseries correlation | 0.54 | 0.44 |
| Mean autocorrelation | 0.85 | 0.84 |
| Mean sensitivity | 0.22 | 0.21 |
| GLK | 0.58 | 0.59 |
| EPS | 0.87 | 0.85 |
3.2 Relationships between HI and climatic factors
When the entire period covered by HI chronology was analysed, the number and composition of climatic factors that were significant for HI of Scots pine differed between the sites (Fig. 3). Annual height increment was mainly related to weather conditions in the year preceding formation of the height increment. Three and five of the tested climatic factors showed significant correlation (p-value < 0.05) with the chronologies, while two and four of these factors showed significant response function coefficients in the western and eastern sites, respectively (Fig. 3). The values of coefficients were rather low and did not exceed ±0.40, suggesting intermediate individual effect of the tested factors on HI.
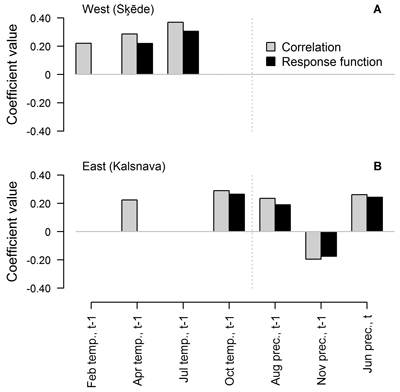
Fig. 3. Statistically significant (p-value < 0.05) Pearson correlation and response function coefficients between climatic factors (mean temperature (temp.) and precipitation sums (prec.) for the preceding (t-1) and current (t) year) and residual chronologies of annual height increment of Scots pine for the western (Šķēde) (A) and eastern (Kalsnava) (B) site. Coefficients are calculated for the entire period covered by the chronologies (from 1906 to 2012 and from 1927 to 2012 in the western and eastern site, respectively).
In the western site, temperature in the previous April and July had a positive effect and the latter appeared as the main predictor of HI, as demonstrated by the significant response function coefficients (0.22 and 0.31, respectively). Temperature in the February of the preceding year showed only significant correlation with HI. In the eastern site, the individual effect of the tested climatic factors was weaker as suggested by lower coefficient values, which did not exceed ±0.29. Temperature in the previous October and precipitation in the previous August and November and in the current June showed significant correlation and response function coefficients. Precipitation in the previous November was the only factor that had a negative effect on HI, but the coefficients values did not exceed 0.20. The effect of April temperature was also observed, but it was weaker since the response function coefficient was non-significant.
The analysis of moving intervals showed that 13 of the tested climatic factors have had significant relationships with HI of Scots pine in at least ten 50-year intervals (Fig. 4). The effect of most of these factors on HI has changed during the 20th century, and accordingly, it has been observed for a short time, with only a few factors showing significant coefficients in more than 20 moving intervals. In the western site, temperature during the dormant period (December t-2, previous February and April) and in the previous October, together with precipitation in the previous March, October and in the current June, has been significant for HI in moving intervals ending in the 1970s and 1980s. However, they were non-significant in the later part of analysed period when temperature in November t-2, in the previous September and in the current May became significant for HI. Temperature in the previous July was the only factor that significantly correlated with HI in all intervals, though response function analysis suggested that this effect has been weakening in the later part of the analysed period.
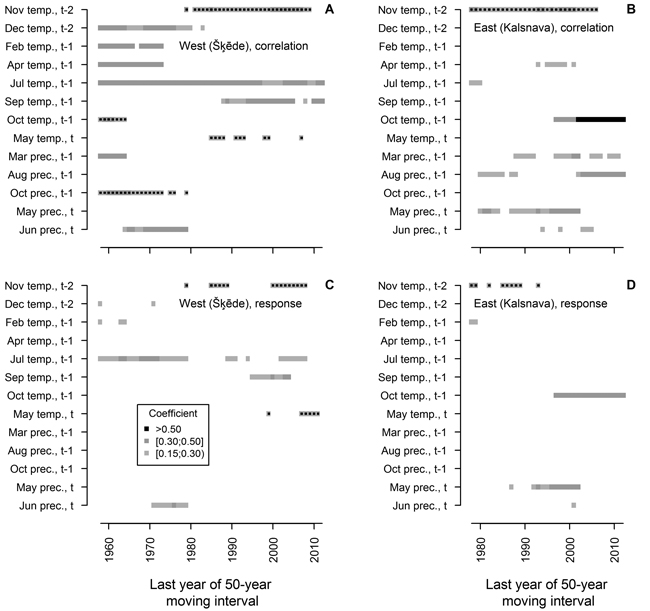
Fig. 4. Statistically significant (p-value < 0.05) Pearson correlation (A and B) and response function (C and D) coefficients calculated between climatic factors (mean temperature (temp.) and precipitation (prec.) for months) and residual chronologies of annual height increment of Scots pine for the western (Šķēde) and eastern (Kalsnava) sites, respectively. Each square represents 50-year moving interval. The dots superimposed on squares represent negative values of coefficients. Note that the length of the analysed periods differs.
Although HI chronology of the eastern site was shorter, a shift in the set of climatic factors significant for HI of Scots pine has been evident throughout the 20th century (Fig. 4). The effect of temperature in November t-2 and precipitation in the current May has been significant during the most of the analysed period, however they have become non-significant in moving intervals ending after 2000. The effect of temperature in the previous October, as portrayed by correlation and response function coefficients, has become significant and strengthened in moving intervals ending after 1997. Precipitation in the previous March and August correlated significantly with HI in scattered intervals throughout the analysed period, with the effect of the latter increasing in moving intervals ending after 2000; however, these factors did not show significant coefficients of response function. Temperature in the previous April and precipitation in the current June correlated significantly with HI in several intervals in the mid-part of analysed period.
4 Discussion
Exact dating of each measurement is crucial for the assessment of relationships between annual variation of tree increment and climatic factors (Fritts 2001); therefore, special attention was paid to crossdating of the time series of HI. Although 25% and 35% of the measured time series showed low agreement and were excluded from the analysis, the individuality of the variation of HI was notable in the crossdated datasets (Fig. 2 A, B), and this might be explained by competition (Rouvinen and Kuuluvainen 1997; Mäkelä and Vanninen 2001). Nevertheless, common signatures of HI were observed amongst most of the trees in several years (Fig. 2 A, B) and EPS values exceeded 0.85 (Table 1), suggesting that datasets reflecting common environmental signals have been produced (Wigley et al. 1984). Mean sensitivity of the time series was intermediate (~ 0.20) and therefore, sufficient for climate-growth analysis (Speer 2010). The time series of HI contained high autocorrelation (> 0.80) (Table 1) suggesting that previous growth had been significant for the current increment, likely via influence on the formation of growth initials (Lanner 1976). The decrease of sample depth in the western site during recent decades (Fig. 2 D) might be explained by cold weather during the sampling, when the wood was fragile and tops were lost during the felling.
The residual chronologies of HI produced from the crossdated time series (Fig. 2 C) had a similar range of index values, as suggested by the values of mean sensitivity (Table 1). The correlation between chronologies was rather low (r = 0.37), portraying marked effect of local factors and suggesting that diverse sets of significant climatic factors have been operational (Wilmking et al. 2004; Andreu et al. 2007; Matisons and Brūmelis 2012). Still, GLK between the chronologies was 0.65, suggesting common tendencies in variation of HI in both sites. The diversification of chronologies that intensified particularly since 1980s (Fig. 2 C) was apparently caused by the divergence of HI patterns amongst trees in the western site (Fig. 2, B), likely in response to warming of the climate (Wilmking et al. 2004; D’Arrigo et al. 2008).
As expected, the sets of climatic factors significant for HI of Scots pine differed between the sites (Fig. 3), likely due to the differences in continentality (Jansons and Baumanis 2005; Matisons and Brūmelis 2012). In the western site where temperature is milder, the temperature in the previous July was the main factor affecting HI of Scots pine (Figs. 3, 4), possibly due to the formation of growth initials (Lanner 1976; Junttila 1986). This has also been observed in Finland (Salminen and Jalkanen 2005). However, response function analysis conducted for moving intervals suggested that this effect has been weakening during the recent decades (Fig. 4), as climate is getting warmer (Lizuma et al. 2007). Temperature in the previous April has also been significant for HI (Fig. 3), possibly due to the extension of the vegetation period when additional nutrients can be assimilated (White et al. 1999), thereby increasing the number of growth initials (Junttila 1986). Correlation analysis conducted for moving intervals suggested that this effect became non-significant in intervals ending after 1973 (Fig. 4 A), also likely due to the warming of climate (Lizuma et al. 2007). In the case of earlier springs, frost can damage buds and impede the positive effect of increased temperature (Gu et al. 2008), which might explain the lack of significant response function coefficients in the earlier part of analysed period (Fig. 4 C). Additionally, analysis of moving intervals showed that low temperature in winter (December and February) which can cause to cold damage (Pearce 2001) has been significant for HI of Scots pine at the beginning of the 20th century (Fig. 4 A, C). The positive effect of temperature in the previous September, which has been apparent during the latter part of the analysed period (Fig. 4 A, C), might be explained by additional assimilation due to a longer vegetation period (White et al 1999). The effect of precipitation in the current June and the negative effect of temperature in the current May (Fig. 4 A, C) suggested that availability of water in early summer has been significant for height growth during some intervals. The negative effect of temperature in the previous October and in November t-2 (Fig. 4 A, C) is difficult to explain, but might be related to increased physiological activity of Scots pine in response to raised temperature, thus depleting nutrient reserves (Ögren et al. 1997). Increased temperature in autumn can also delay cold hardening (Repo et al. 2000), thus increasing susceptibility to damage from sudden shifts of temperature (Pearce 2001; Hänninen 2006).
Under the harsher climate of the eastern site, there has been a clear shift of significant climatic factors, compared to that of the western site (Fig. 4 C, D), since none of the factors have been significant during the entire period. This also might explain the lower values of correlation and response function coefficients observed when the entire chronology was analysed (Fig. 3 B). Although shoot elongation is considered to depend on current assimilation (Ericsson 1978; Hansen and Beck 1994; Lippu 1998; von Felten et al. 2007), our results suggested that alternative response mechanisms might be possible. Generally, HI was affected by weather conditions in the year preceding growth, but later in the season (after July), after the cessation of height growth of Scots pine (Jansons et al. 2011), thus suggesting that nutrient reserves have apparently had a certain effect on shoot elongation. This is stressed by the significant effect of temperature in the previous October, which has become the main climatic factor limiting HI (Fig. 4 B, D). Such relationships might be explained by the extension of the vegetation period due to increased temperature in autumn and additional assimilation of reserve nutrients (White et al. 1999), thus increasing the vigour of trees (Pallardy 2008) and successive growth. Increased vigour enhances cold tolerance and overwintering (Pearce 2001), especially when long thaws, which foster susceptibility to frost damage (Hänninen 2006), are becoming more frequent (Avotniece et al. 2010). The linkage of HI to nutrient reserves also seems to be influenced by the effect of precipitation in the previous August (Figs. 3, 4 B) which might be explained by decreased assimilation due to water deficit (Pallardy 2008), as observed for radial growth of Scots pine in Central Europe (Oberhuber et al. 1998; Lebourgeois 2010). The availability of water during shoot elongation also has affected HI as suggested by significant correlation with precipitation in the current May and June (Fig. 4 B). These factors became non-significant in the later part of the analysed period that might be explained by the shifting of limiting factors (Speer 2010). However, considering that only one generation of trees were analysed, the shift of significant climatic factors might be also age-related (Carrer and Urbinati 2004).
5 Conclusions
Height increment of Scots pine growing in stands in western and eastern Latvia showed common tendencies during the 20th century. However, the suite of climatic factors affecting HI differed between the sites, presumably due to the differences in continentality, as previously observed in growth of other species. In both sites, the suite of significant climatic factors has changed during the studied period, which might be explained by the warming of the climate. In the maritime western site, HI was mainly affected by temperature in July, but temperature in the dormant period and spring has been significant at the beginning of the 20th century. The effect of temperature in September has become significant since the mid-part of the 20th century, suggesting influence of the length of the vegetation period on HI. This relationship suggests that climate is becoming optimal for height growth of Scots pine in the western site. The effect of the length of vegetation season was even more apparent in the eastern site where the effect of temperature in October has emerged and intensified in the second part of the 20th century, becoming the main factor affecting HI. Still, the positive effect of precipitation in May, June and August has been observed, suggesting that availability of water has been affecting height growth.
Mainly, HI of Scots pine was related to weather conditions prior to height growth, which likely affected the number of growth initials. Although shoot growth of Scots pine is considered to depend on the number of growth initials and on current assimilation, the observed relationships with climatic factors after the formation of the terminal bud suggest that nutrient reserves also influences formation of HI.
Acknowledgements
The study was supported by Forest Competence Centre (ERAF) project “Methods and technologies for increasing forest capital value” (No. L-KC-11-0004). Didzis Elferts helped with the arrangement of climatic data; Andis Adamovičs, Juris Kalniņš, Kārlis Taukačš and Juris Katrēvičs much helped during the sampling. Sabina Khan revised the language of manuscript.
References
Andreu L., Guiterrez E., Macias M., Ribas M., Bosch O., Camarero J.J. (2007). Climate increases regional tree-growth variability in Iberian pine forests. Global Change Biology 13: 804–815. http://dx.doi.org/10.1111/j.1365-2486.2007.01322.x.
Avotniece Z., Rodinov V., Lizuma L., Briede A., Kļaviņš M. (2010). Trends in frequency of extreme climate events in Latvia. Baltica 23: 135–148.
Biondi F., Waikul K. (2004). DENDROCLIM2002: a C++ program for statistical calibration of climate signals in tree ring chronologies. Computers and Geosciences 30: 303–311. http://dx.doi.org/10.1016/j.cageo.2003.11.004.
Briede A., Lizuma L. (2007). Long-term variability of precipitation in the territory of Latvia. In: Kļaviņš M. (ed.). Climate change in Latvia. University of Latvia, Rīga. p. 35–45.
Bunn A.G. (2008). A dendrochronology program library in R (dplR). Dendrochronologia 26: 115–124. http://dx.doi.org/10.1016/j.dendro.2008.01.002.
Bušs K. (1976). Basis of forest classification in SSR of Latvia. LRZTIPI, Riga. 73 p. [In Latvian].
Carrer M., Urbinati C. (2004). Age-dependant tree-ring growth response to climate in Larix decidua and Pinus cembra. Ecology 85: 730–740. http://dx.doi.org/10.1890/02-0478.
Cook E.R., Holmes R.L. (1996). Guide for computer program ARSTAN. In: Grissino-Mayer H.D., Holmes R.L., Fritts H.C. (eds.). The international tree-ring data bank program library version 2.0 user’s manual. University of Arizona, Tucson. p. 75–87.
D’Arrigo R., Wilson R., Liepert B., Cherubini P. (2008). On the ‘divergence problem’ in northern forests: a review of the tree-ring evidence and possible causes. Global and Planetary Change 60: 289–305. http://dx.doi.org/10.1016/j.gloplacha.2007.03.004.
Dobbertin M., Eilmann B., Bleuler P., Giuggiola A., Graf-Pannatier E., Landolt W., Schleppi P., Rigling A. (2010). Effect of irrigation on needle morphology, shoot and stem growth in a drought-exposed Pinus sylvestris forest. Tree Physiology 30: 346–360. http://dx.doi.org/10.1093/treephys/tpp123.
Ericsson A. (1978). Seasonal changes in translocation of 14C from different age classes of needles on 20-year-old Scots pine trees (Pinus silvestris). Physiologia Plantarum 43: 351–358. http://dx.doi.org/10.1111/j.1399-3054.1978.tb01593.x.
EUFORGEN (2009). Distribution maps: Pinus sylvestris. http://www.euforgen.org/fileadmin/templates/euforgen.org/upload/Documents/Maps/JPG/Pinus_sylvestris.jpg. [Cited 01 Aug 2014].
Fritts H.C. (2001). Tree-rings and climate. The Blackburn Press, Caldwell. 567 p.
Grissino-Mayer H.D. (2001). Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree-Ring Research 57: 205–221.
Gu L., Hanson P.J., Post W.M., Kaiser D.P., Yang B., Nemani R., Pallardy S.G., Meyers T. (2008). The 2007 Eastern US spring freeze: increased cold damage in a warming world? BioScience (2008) 58(3): 253-262. http://dx.doi.org/10.1641/B580311.
Hanewinkel M., Cullmann D.A., Schelhaas M.J., Nabuurs G.J. (2012). Climate change may cause severe loss in the economic value of European forest land. Nature Climate Change 3: 203–207. http://dx.doi.org/10.1038/nclimate1687.
Hänninen H. (2006). Climate warming and the risk of frost damage to boreal forest trees: identification of critical ecophysiological traits. Tree Physiology 26: 889–898. http://dx.doi.org/10.1093/treephys/26.7.889.
Hansen J., Beck E. (1994). Seasonal changes in the utilization and turnover of assimilation products in 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees – Structure and Function 8: 172–182. http://dx.doi.org/10.1007/BF00196844.
Harris I., Jones P.D., Osborn T.J., Lister D.H. (2014). Updated high-resolution grids of monthly climatic observations – the CRU TS3.10 Dataset. International Journal of Climatology 34: 623–642. http://dx.doi.org/10.1002/joc.3711.
Hickler T., Vohland K., Feehan J., Miller P.A., Smith B., Costa L., Giesecke T., Fronzek S., Carter T.R., Cramer W., Kuhn I., Sykes M.T. (2012). Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model. Global Ecology and Biogeography 21: 60–63. http://dx.doi.org/10.1111/j.1466-8238.2010.00613.x.
Iverson L.R., Prasad A.M. (1998). Predicting abundance of 80 tree species following climate change in eastern United States. Ecological Monographs 68: 465–485. http://dx.doi.org/10.1890/0012-9615(1998)068[0465:PAOTSF]2.0.CO;2.
Jansons Ā., Baumanis I. (2005). Growth dynamics of Scots pine geographical provenances in Latvia. Baltic Forestry 11: 29–37.
Jansons Ā., Krišāns O., Jansons J. (2011). Seasonal height growth dynamics of Scots pine (Pinus sylvestris L.). Mežzinātne 23: 15–24. [In Latvian].
Jansons Ā., Matisons R., Baumanis I, Puriņa L. (2013). Effect of climatic factors on height increment of Scots pine in experimental plantation in Kalsnava, Latvia. Forest Ecology and Management 306: 185–191. http://dx.doi.org/10.1016/j.foreco.2013.06.039.
Junttila O. (1986). Effect of temperature on shoot growth in northern provenances of Pinus sylvestris L. Tree Physiology 1: 185–192. http://dx.doi.org/10.1093/treephys/1.2.185.
Kaya Z., Sewell M.M., Neale D.B. (1999). Identification of quantitative trait loci influencing annual height and diameter-increment growth in loblolly pine (Pinus taeda L.). Theoretical and Applied Genetics 98: 586–592. http://dx.doi.org/10.1007/s001220051108.
Kellomäki S., Ikonen V.P., Peltola H., Kolström T. (1999). Modelling the structural growth of Scots pine with implications for wood quality. Ecological Modelling 122: 117–134. http://dx.doi.org/10.1016/S0304-3800(99)00086-1.
Krams M., Ziverts A. (1993). Experiments of conceptual mathematical groundwater dynamics and runoff modelling in Latvia. Nordic Hydrology 24: 243–262. http://dx.doi.org/10.2166/nh.1993.016.
Lanner R.M. (1976). Patterns of shoot development in Pinus and their relationship to growth potential. In: Cannell M.G.R., Last F.T. (eds.) Tree physiology and yield improvement. Academic press, London. p. 223–243.
Lebourgeois F. (2010). Sensitivity of French temperate coniferous forests to climate variability and extreme events (Abies alba, Picea abies and Pinus sylvestris). Journal of Vegetation Science 21: 364–376. http://dx.doi.org/10.1111/j.1654-1103.2009.01148.x.
Lippu J. (1998). Redistribution of 14C-labelled reserve carbon in Pinus sylvestris seedlings during shoot elongation. Silva Fennica 32: 3–10. http://dx.doi.org/10.14214/sf.696.
Little C.H.A., Pharis R.P. (1995). Hormonal control of radial and longitudinal growth in tree stem. In: Gartner B. (ed.). Plant stems: physiology and functional morphology. Academic Press, San Diego. p. 282–319.
Lizuma L., Kļaviņš M., Briede A., Rodinovs V. (2007). Long-term changes of air temperature in Latvia. In: Kļaviņš M. (ed.). Climate change in Latvia. University of Latvia, Riga. p. 11–20.
Maiorano L., Cheddadi R., Zimmermann N.E., Pellisser L., Petitpierre B., Pottier J., Laborde H., Hurdu B.I., Pearman P.B., Psomas A., Singarayer J.S., Broenimman O., Vittoz P., Dubuis A., Edwards M.E., Binney H.A., Guisan A. (2013). Building the niche through time: using 13,000 years of data to predict the effects of climate change on three tree species in Europe. Global Ecology and Biogeography 22: 302–317. http://dx.doi.org/10.1111/j.1466-8238.2012.00767.x.
Mäkelä A., Vanninen P. (2001). Vertical structure of Scots pine crowns in different age and size classes. Trees – Structure and Function 15: 385–392. http://dx.doi.org/10.1007/s004680100118.
Matisons R., Brūmelis G. (2012). Influence of climate on tree-ring and earlywood vessel formation in Quercus robur in Latvia. Trees – Structure and Function 26: 1251–1266. http://dx.doi.org/10.1007/s00468-012-0701-z.
McCarroll D., Jalkanen R., Hicks S., Tuovinen M., Gagen M., Pawellek F., Eckstein D., Schmitt U., Autio J., Heikkinen O. (2003). Multiproxy dendroclimatology: a pilot study in northern Finland. Holocene 13: 826–838. http://dx.doi.org/10.1191/0959683603hl668rp.
Mutke S., Gordo J., Climent J., Gil L. (2003). Shoot growth and phenology modelling of grafted Stone pine (Pinus pinea L.) in Inner Spain. Annals of Forest Science 60: 527–537. http://dx.doi.org/10.1051/forest:2003046.
Neimane U., Veinberga I., Ruņģis D. (2009). Phenotypic and genetic aspects of geographical differences in Scots pine populations of Latvia. Mežzinātne 20: 3–15. [In Latvian].
Oberhuber W., Stumböck M., Kofler W. (1998). Climate-tree-growth relationships of Scots pine stands (Pinus sylvestris L.) exposed to soil dryness. Trees – Structure and Function 13: 19–27. http://dx.doi.org/10.1007/PL00009734.
Ögren E., Nilsson T., Sundblad L.G. (1997). Relationship between respiratory depletion of sugars and loss of cold hardiness in coniferous seedlings overwintering at raised temperatures: indications of different sensitivities of spruce and pine. Plant, Cell and Environment 20: 247–253. http://dx.doi.org/10.1046/j.1365-3040.1997.d01-56.x.
Pallardy S.G. (2008). Physiology of woody plants. Third ed. Elsevier, London. 454 p.
Pearce R.S. (2001). Plant freezing and damage. Annals of Botany 87: 417–424. http://dx.doi.org/10.1006/anbo.2000.1352.
Pensa M., Salminen H., Jalkanen R. (2005). A 250-year-long height-increment chronology for Pinus sylvestris at the northern coniferous timberline: a novel tool for reconstructing past summer temperatures? Dendrochronologia 22: 75–81. http://dx.doi.org/10.1016/j.dendro.2005.02.005.
R Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. [Cited 1 Aug 2014].
Reich P.B., Oleksyn J. (2008). Climate warming will reduce growth and survival of Scots pine except in the far north. Ecology Letters 11: 588–597. http://dx.doi.org/10.1111/j.1461-0248.2008.01172.x.
Repo T., Zhang G., Ryyppö A., Rikala R., Vuorinen M. (2000). The relation between growth cessation and frost hardiness in Scots pine of different origins. Trees – Structure and Function 14: 456–464. http://dx.doi.org/10.1007/s004680000059.
Rouvinen S., Kuuluvainen T. (1997). Structure and asymmetry of tree crowns in relation to local competition in a natural mature Scots pine forest. Canadian Journal of Forest Research 27: 890–902. http://dx.doi.org/10.1139/x97-012.
Ryan M.G., Yoder B.J. (1997). Hydraulic limits to tree height and tree growth. Bioscience 47: 235–242. http://www.jstor.org/stable/1313077.
Salminen H., Jalkanen R. (2005). Modelling the effect of temperature on height increment of Scots pine at high latitudes. Silva Fennica 39: 497–508. http://dx.doi.org/10.14214/sf.362.
Salminen H., Jalkanen R. (2007). Intra-annual height increment of Pinus sylvestris at high latitudes in Finland. Tree Physiology 27: 1347–1353. http://dx.doi.org/10.1093/treephys/27.9.1347.
Savill P., Evans J., Auclair D., Falck J. (1997). Plantation silviculture in Europe. Oxford University Press, Oxford. 308 p.
Speer J.H. (2010). Fundamentals of tree-ring research. The University of Arizona Press, Tucson. 333 p.
Stott P., Loehle C. (1998). Height growth rate tradeoffs determine northern and southern range limits for trees. Journal of Biogeography 25: 735-742. http://dx.doi.org/10.1046/j.1365-2699.1998.2540735.x.
Thabeet A., Vennetier M., Gadbin-Henry C., Dendelle N., Roux M., Caraglio Y., Vila B. (2009). Response of Pinus sylvestris L. to recent climatic events in the French Mediterranean region. Trees – Structure and Function 23: 843–853. http://dx.doi.org/10.1007/s00468-009-0326-z.
Volosyanchuk R.T. (2002). Pinus sylvestris. In: Burdon R.D. (ed.). Pines of silvicultural importance. CABI Publishing, London. p. 449–466.
von Felten S., Hättenschwiler S., Saurer M., Siegwolf R. (2007). Carbon allocation in shoots of alpine treeline conifers in a CO2 enriched environment. Trees – Structure and Function 21: 283–284. http://dx.doi.org/10.1007/s00468-006-0118-7.
White M.A., Running S.W., Thornton P.E. (1999). The impact of growing-season length variability on carbon assimilation and evapotranspiration over 88 years in the eastern US deciduous forest. Journal of International Biometeorology 42: 139–145. http://dx.doi.org/10.1007/s004840050097.
Wigley T.M.L., Briffa K.R., Jones P.D. (1984). On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. Journal of Climate and Applied Meteorology 23: 201–213. http://dx.doi.org/10.1175/1520-0450(1984)023<0201:OTAVOC>2.0.CO;2.
Wilmking M., Juday G.P., Barber V.A., Zald H.J. (2004). Recent climate warming forces contrasting growth responses of white spruce at treeline in Alaska through temperature thresholds. Global Change Biology 10: 1724–1736. http://dx.doi.org/10.1111/j.1365-2486.2004.00826.x.
Total of 60 references

